Abstract
Purpose
This study investigated the inhibitory effects of Paclitaxel by altering tubulin assembly and cisplatin exposure by binding DNA of the lens epithelial cells (LECs) during epithelial cell cultures in the capsular bag model.
Methods
In the capsular bag model, the LECs were cultured with exposure to Paclitaxel (1, 10, 100 nM) and Cisplatin (1, 10, 100 µM) for 3 min. The effect of Paclitaxel and Cisplatin was analyzed by observing the cell number of fibroblasts per field, Western blots for type IV collagen, TUNEL assay and the Proliferating Cell Nuclear Antigen (PCNA) and Bromodeoxyuridine (BrdU) incorporated proliferating cells.
Results
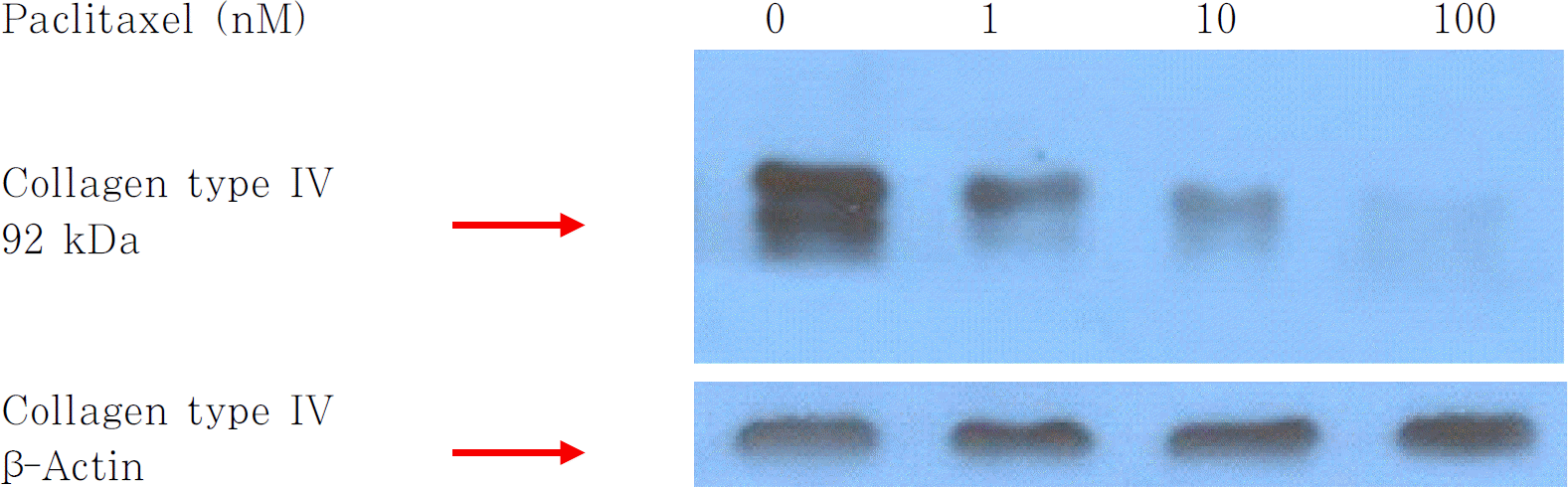
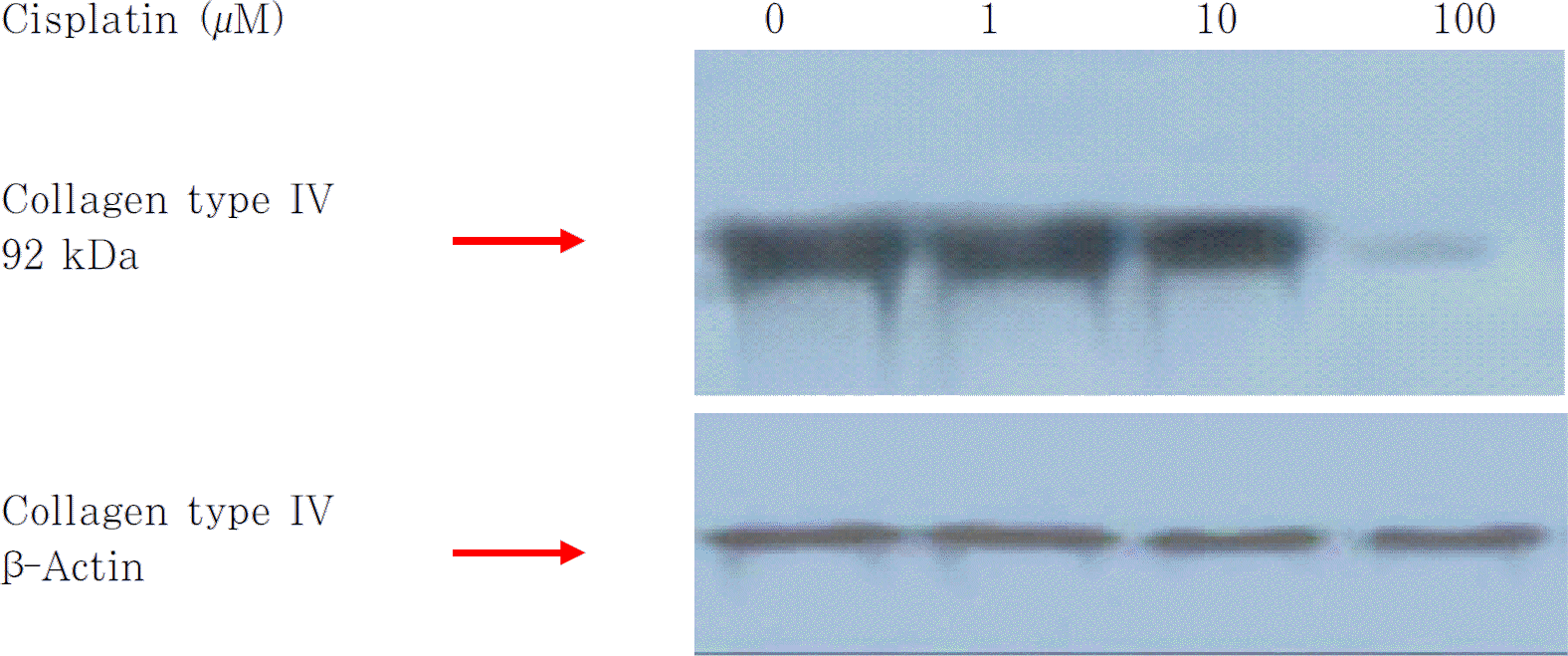
An increase in concentration of Paclitaxel and Cisplatin resulted in a decrease in the number of fibroblasts and spindle-shaped cells. The number of proliferating cells showing PCNA positivity and BrdU incorporation in the nuclei was decreased in a dose dependent manner by treatments of Paclitaxel and Cisplatin. Expression of type IV collagen also decreased after treatment with these two agents. Results of the TUNEL assay showed no change in the apoptosis of cells with regard to an increase in concentration of Paclitaxel and Cisplatin.
Go to : 
References
1. Legler UF, Apple DJ, Assia EI, et al. Inhibition of posterior capsule opacification: The effect of colchicine in a sustained drug delivery system. J Cataract Refract Surg. 1993; 19:462–70.

2. Pandey SK, Cochener B, Apple DJ, et al. Intracapsular ring 5-fluorouracil delivery system for the prevention of posterior capsule opacification in rabbit. J Cataract Refract Surg. 2002; 25:139–48.
3. Jordan JF, Kociock N, Grisanti S. Specific feature of apoptosis in human lens epithelial cells induced by Mitomycin C in vitro. Greafes Arch Clin Exp Ophthalmol. 2001; 239:613–8.
4. Park TS, Tea JS, Park SM, Oh JH. Inhibitory effects of imatinib mesylate on transdifferentiation of lens epithelial cells into fibroblast. J Korean Ophthalmol Soc. 2005; 46:257–70.
5. Kim SJ, Byun GD, Tae JS, et al. The Effect of Imatinib (Glivec) on The Proliferation of Lens Epithelial Cells and The Activities of Growth Factors. J Korean Ophthalmol Soc. 2003; 44:2637–51.
6. Oh JH, Byun KD, Yun YS. Inhibitory effect of genistein on the proliferation of lens epithelial cells. J Korean Ophthalmol Soc. 2001; 42:865–77.
7. Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992; 37:73–116.

8. Cobo LM, Ohsawa E, Chandler D, et al. Pathogenesis of capsular opacification after extracapsular cataract extraction: An animal model. Ophthalmology. 1984; 91:857–63.
9. Bertelmann E, Kojetinsky C. Posterior capsule opacification and anterior capsule opacification. Curr Opin Ophthalmol. 2001; 12:35–40.

10. Kim HG, Kang TJ, Lee SK, Bae JH. A study on mitomycin C induced damage to the iris and ciliary body of cats. J Korean Ophthalmol Soc. 2005; 36:486–91.
11. Do SJ, Kang SJ, Lee JH, et al. The Effect of the Remnants after Cataract Extraction on the Lens Epithelial Cell Culture. J Korean Ophthalmol Soc. 2002; 43:872–82.
12. Caplow M, Shanks J, Ruhlen R. How taxol modulates microtuble disassembly. J Biol Chem. 1994; 269:23399–402.
13. Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother. 2002; 3:755–66.
15. Miranda S, Malan Borel I, Margni R. Altered modulation of the in vitro antibody synthesis by placental factors from the CBA/J × DBA/2 abortion-prone mating combination. Am J Reprod Immunol. 1998; 39:341–9.

16. Joel GH, Lee EL, Alfred GG. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed.U S A McGraw-Hill;2001. p. 1260–1.
17. Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006; 355:2113–24.

18. Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation. 2004; 109:1948–54.
19. Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993; 328:184–93.

20. Novakova O, Vrana O, Kiseleva VI, Brabec V. DNA interaction of antitumor platinum (IV) complexes. Eur J Biochem. 1995; 228:616–24.
21. Rozencweig M, Von Hoff DD, Muggia FM. Investigational chemotherapeutic agents in head and neck cancer. Semin Oncol. 1977; 4:425–9.
22. Fichtinger-Schepman AMJ, van Oosterom AT, Lohman PHM, Berends F. Cis-diammine-dichloroplatinum (II) – induced DNA adducts in peripheral leukocytes from seven cancer patients:quantitative immunochemical detection of the adduct induction and removal after a single dose of cis-diammine-dichloroplatinum (II). Cancer Res. 1987; 47:3000–4.
23. Bernal-Mendez E, Boudvillan M, Gonzalez-Vilchez F, Leng M. Chemical versatility of transplatin monofunctional adducts within multiple site-specifically platinated DNA. Biochemistry. 1997; 36:7281–7.

24. DeVita VT Jr, Hubbard SM. NCI's breast cancer clinical alert: rationale and results. Resid Staff Physician. 1989; 15:35:49–55.
26. Khan A, Hill JM. Suppression of graft-versus-host reaction by cis-platinum. II. Diaminodichloride. Transplantation. 1972; 13:55–7.

27. Brambilla G, Cavanna M, Maura A. Effect of cis-diamminedichloroplatinum (NSC-119875) on the allograft reaction in mice. Cancer Chemother Rep. 1974; 58:633–6.
28. Beck DJ, Brubaker RR. Mutagenic properties of cis-plantinum(II) diammino-dichloride in Escherichia coli. Mutat Res. 1975; 27:181–9.
29. Kurki P, Vanderlaan M, Dolbeare F, et al. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986; 166:209.

30. Tahan SR, Neuberg DS, Dieffenbach A, Yacoub L. Prediction of early relapse and shortened survival in patient with breast cancer by proliferating cell nuclear antigen score. Cancer. 1993; 71:3552.
Go to : 
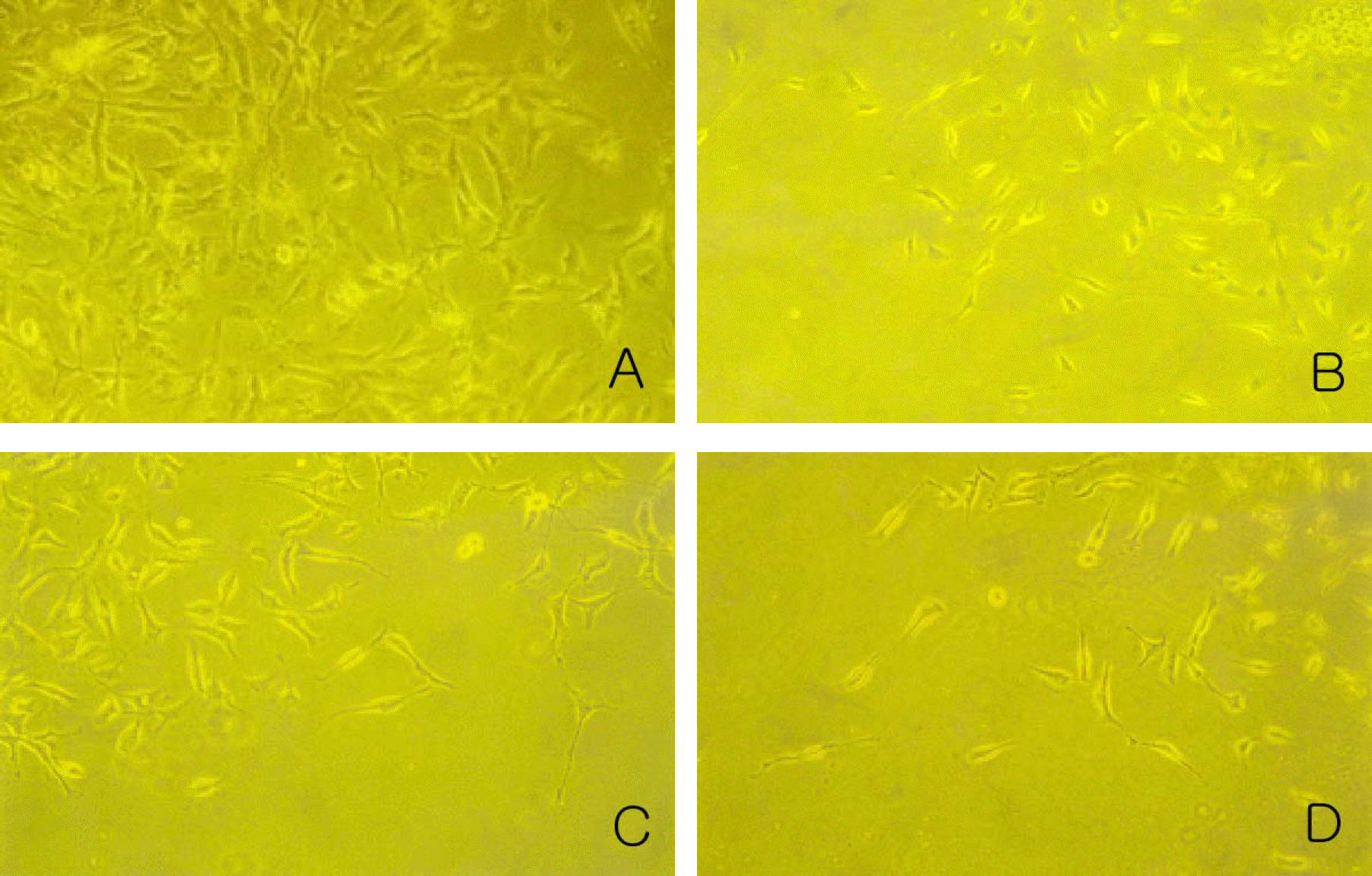
 | Figure 1.Morphological profiles of the transdifferentiated cells of the Paclitaxel-treated group (experimental group). Magnification: 200x. The group treated with 1 nM (B), 10 nM (C) and 100 nM Paclitaxel (D) showed slightly decreased cell number than control group (A). |
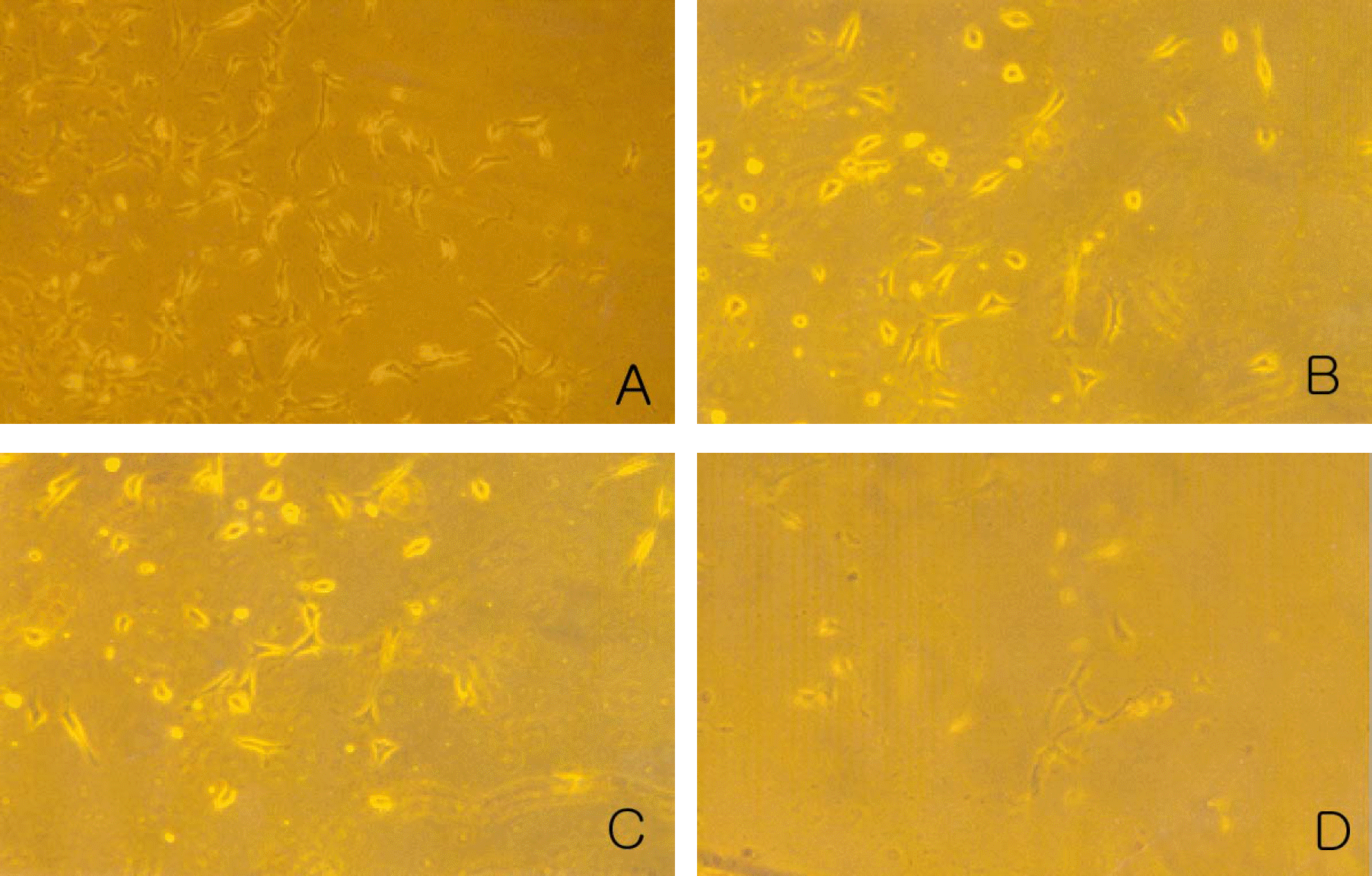
 | Figure 2.Morphological profiles of the transdifferentiated cells in the Cisplatin-treated group (experimental group II). Magnification: 200×. The group treated with 1 µM (B), 10 µM (C) and 100 µM Cisplatin (D) showed slightly decreased cell number than control group (A). |
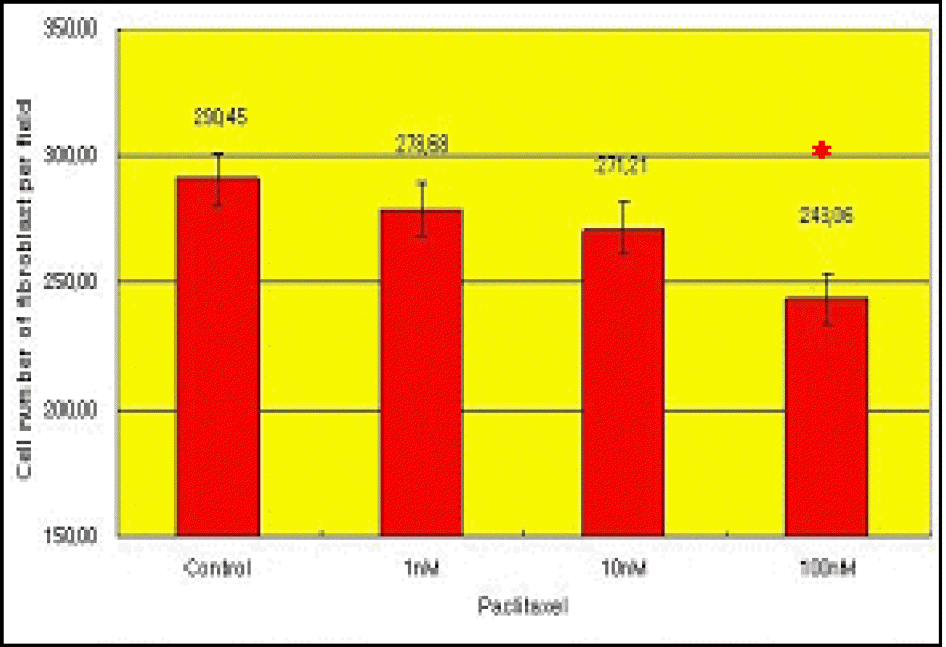
 | Figure 3.The cell number of fibroblast per microscopic field during culture in experimental group I. The cell number was decreased by treatment of Paclitaxel in dose- * Significant decrease of the cell number dependent manner. in 100 nM Paclitaxel-treated group. (p<0.05) |
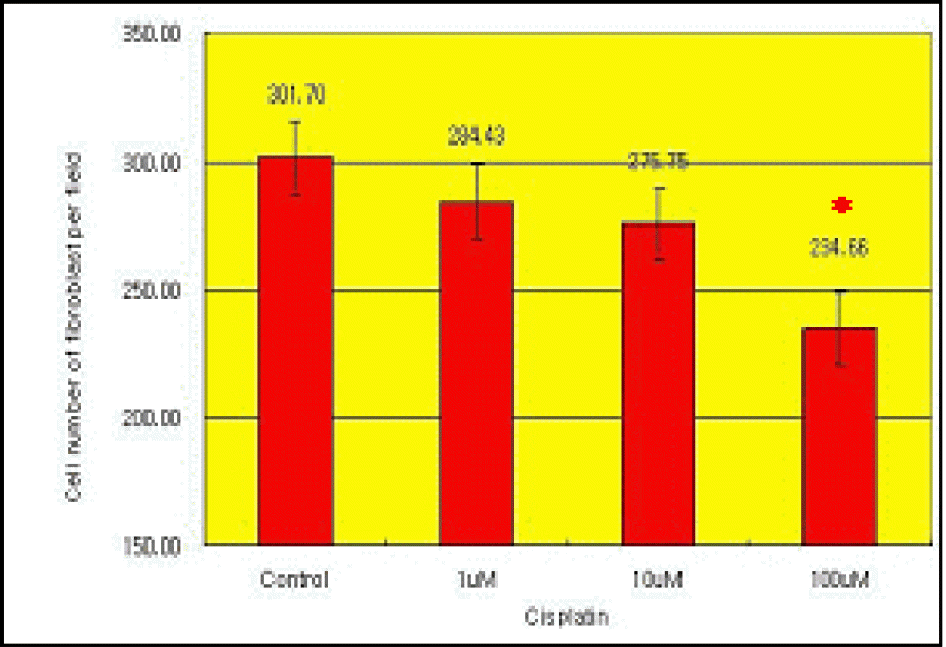
 | Figure 4.The cell number of fibroblast per microscopic field during culture in experimental group II. The cell number was decreased by treatment of Cisplatin in * Significant decrease of the cell dose-dependent manner. number in 100 µM Cisplatin-treated group. (p<0.05) |
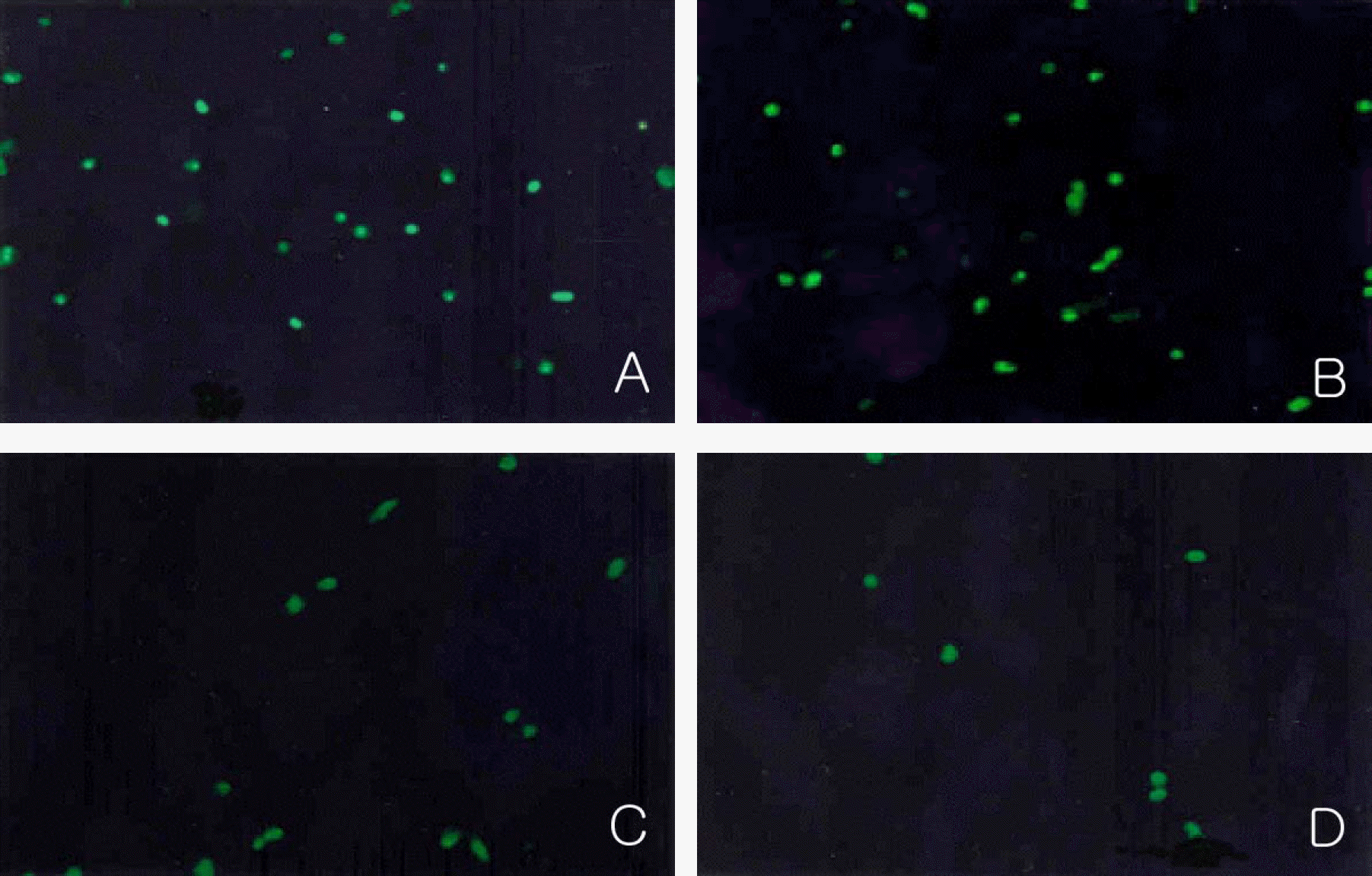 | Figure 5.Immunocytochemical detection of the incorporated BrdU in the nuclei of proliferating cells at day 7 in experimental group I. Magnification: 200×. The number of cells incorporated with BrdU in their nuclei decreased according to increasing concentrations of 1 nM (B), 10 nM (C) and 100 nM Paclitaxel (D) than control group (A). |
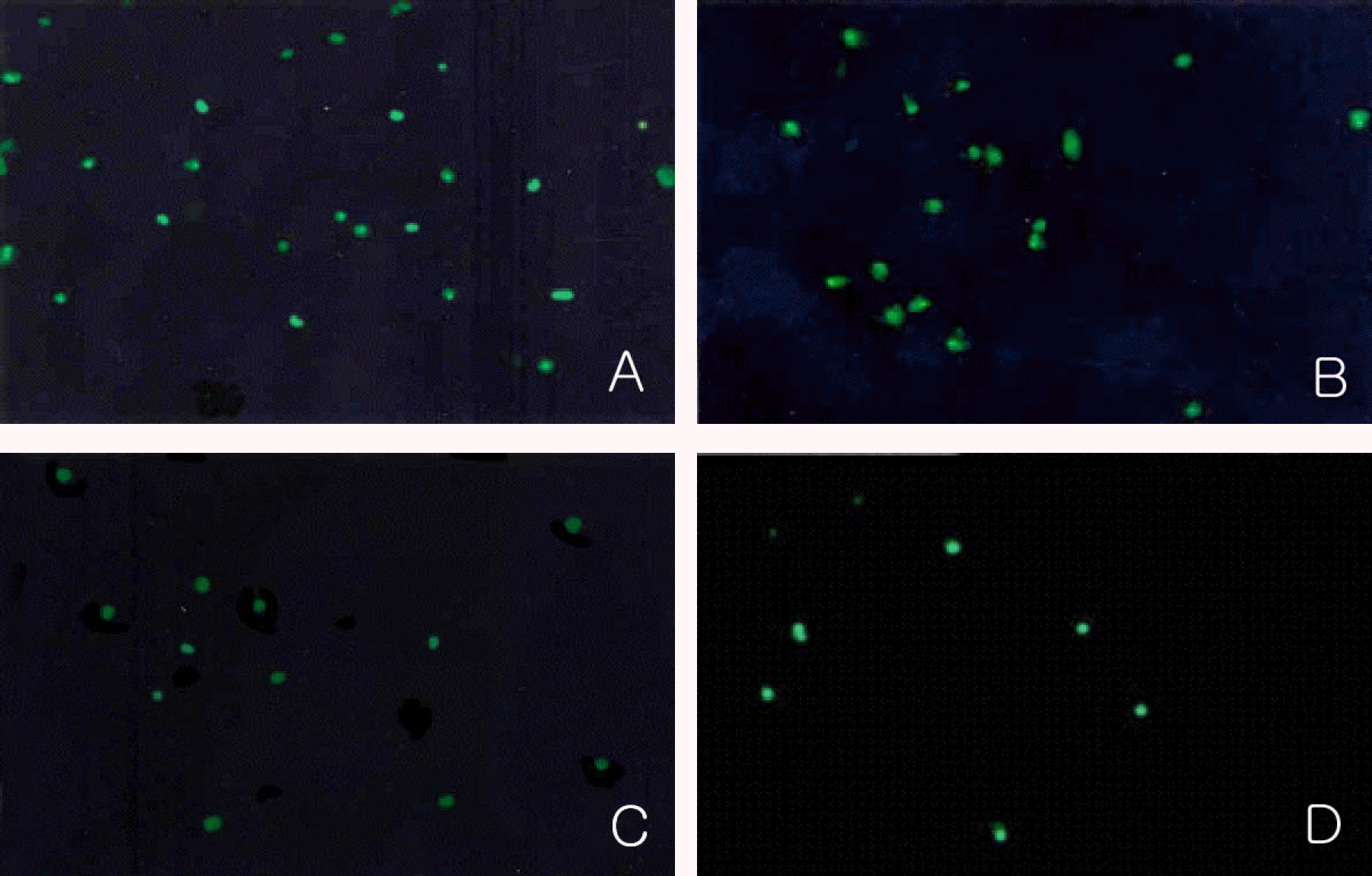 | Figure 6.Immunocytochemical detection of the incorporated BrdU in the nuclei of proliferating cells at day 7 in experimental group II. Magnification: 200×. The number of cells incorporated with BrdU in their nuclei decreased according to increasing concentrations of 1 µM (B), 10 µM (C) and 100 µM Cisplatin (D) than control group (A). |
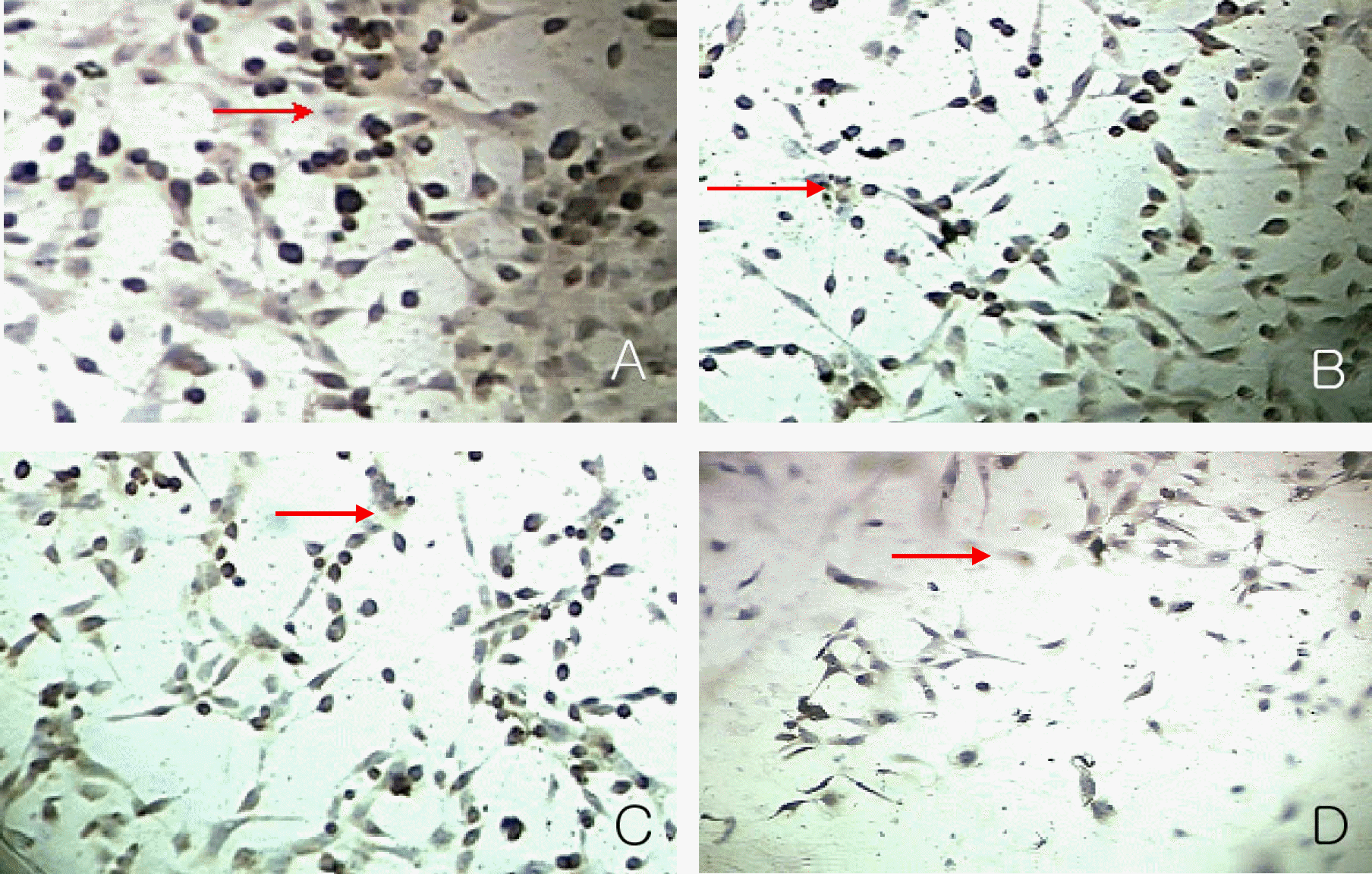 | Figure 7.Immunocytochemical detection of the PCNA in the nuclei of proliferating cells at day 7 in experimental group I. Magnification: 200× The number of PCNA positive cells decreased according to increasing concentrations of 1 nM (B), 10 nM (C) and 100 nM Paclitaxel (D) than control group (A). |
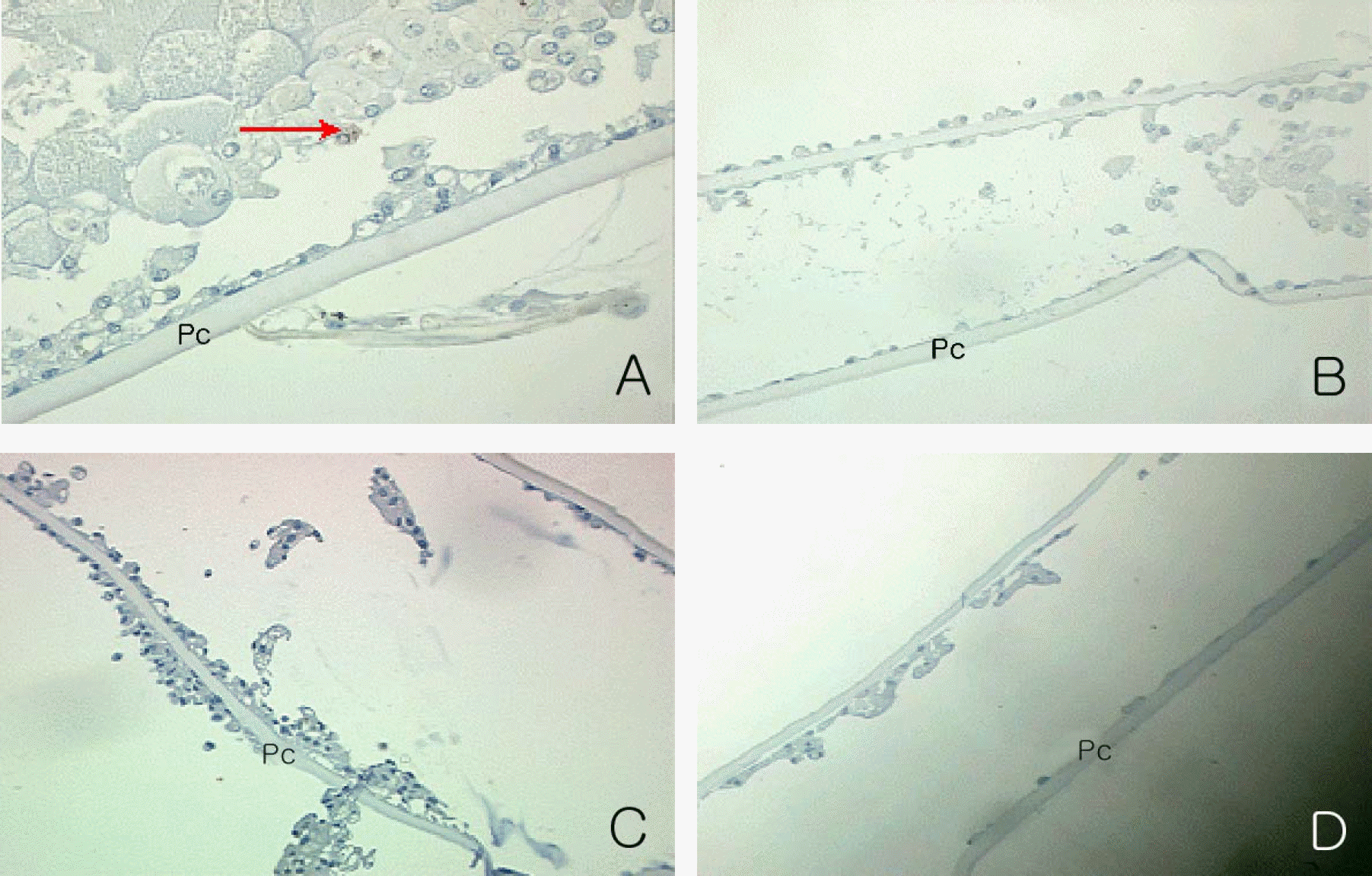 | Figure 8.Immunocytochemical detection of the PCNA positive proliferating cells on the cross section of cultured capsular bag at day 7 in experimental group I. PCNA positive cell (red arrow, a reddish brown diffuse or granular nucleus staining) was noted in control (A) (×200). PCNA positive cell was not noted in 1 nM (B), 10 nM (C) and 100 nM Paclitaxel (D) (×100). |
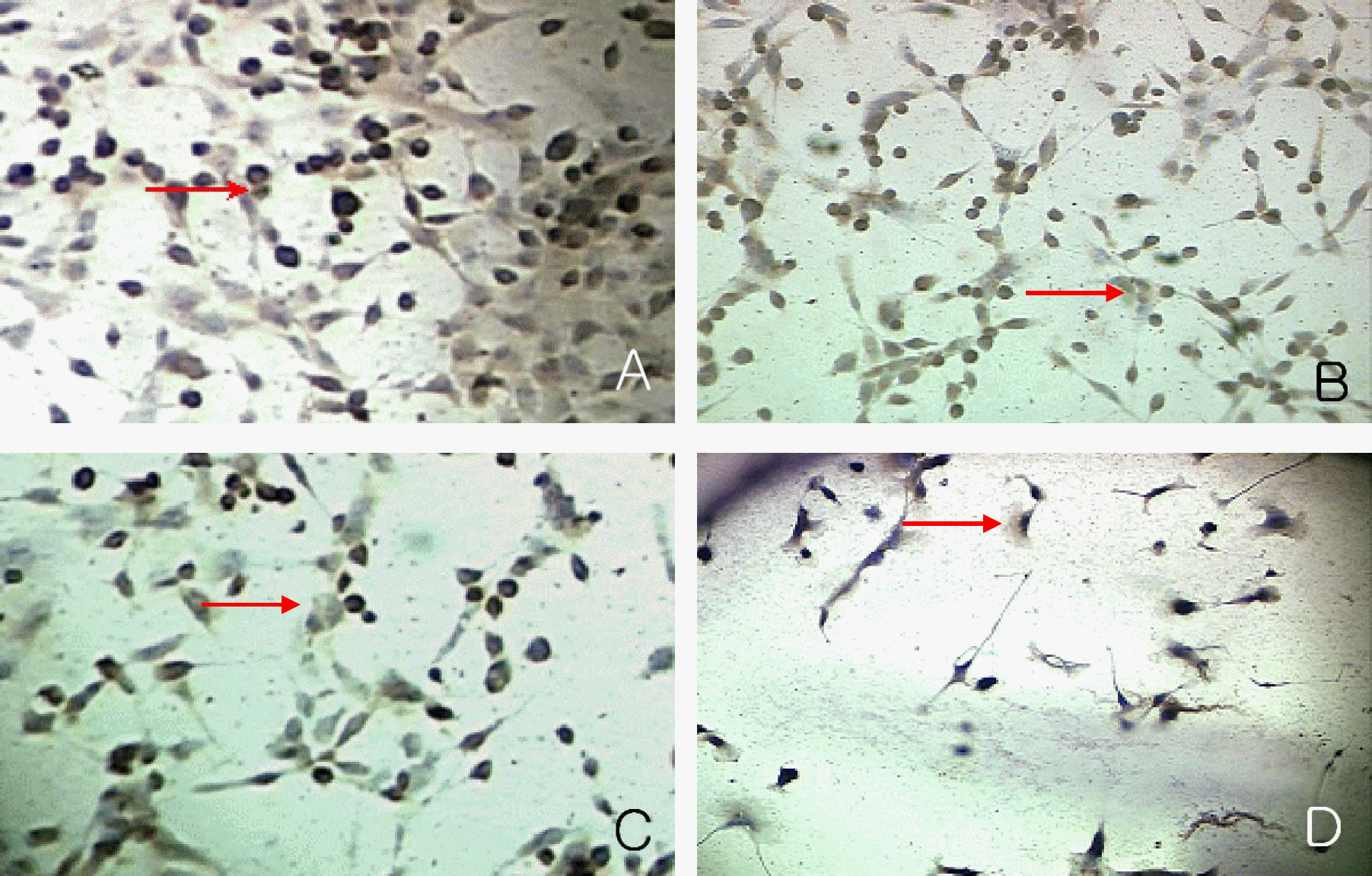 | Figure 9.Immunocytochemical detection of the PCNA in the nuclei of proliferating cells at day 7 in experimental group II. Magnification:200× The number of PCNA positive cells(a reddish brown diffuse or granular nucleus staining) decreased according to increasing concentrations of 1 µM (B), 10 µM (C) and 100 µM Cisplatin (D) than control group (A). |
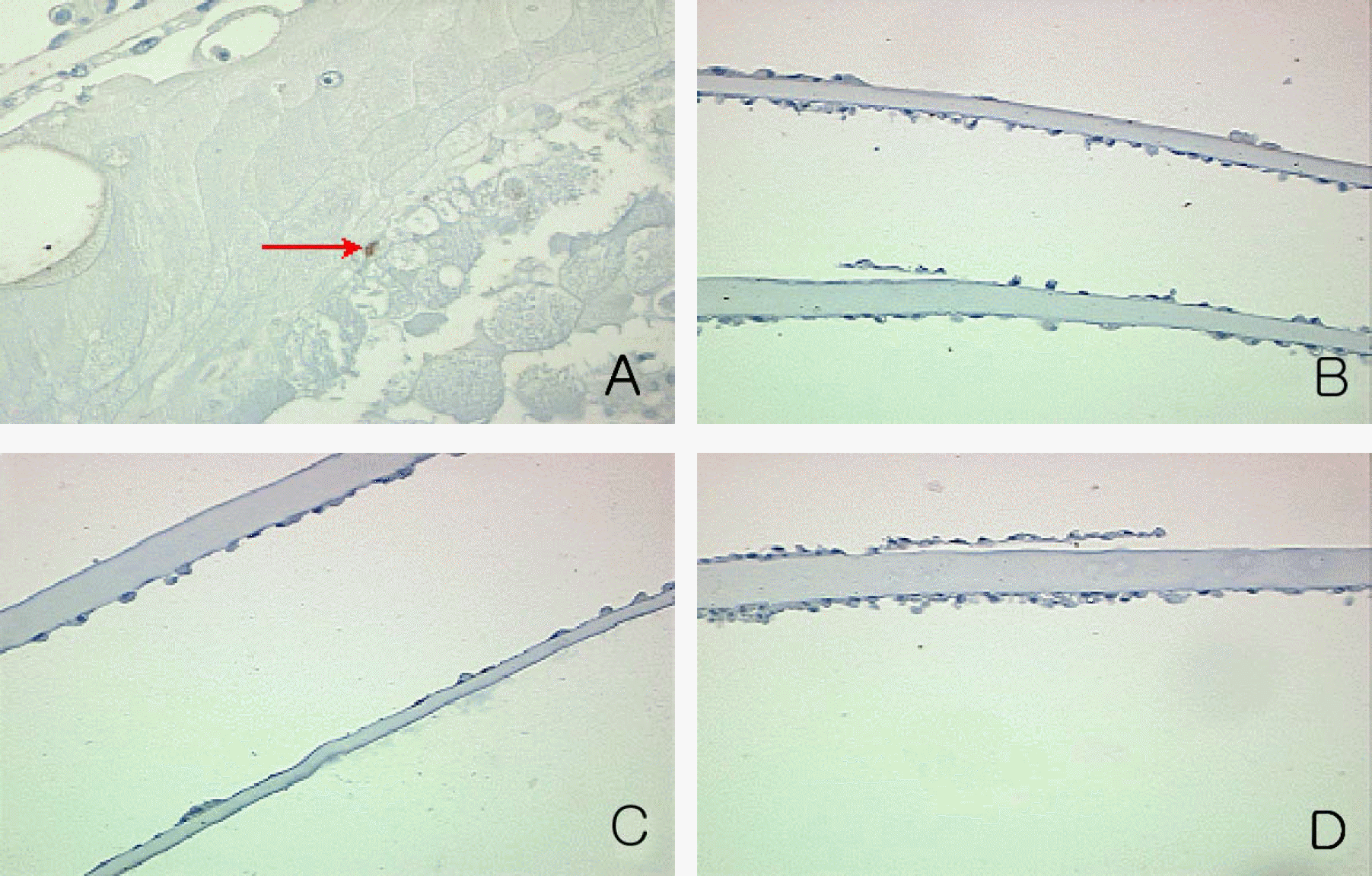 | Figure 10.Immunocytochemical detection of the PCNA positive proliferating cells on the cross section of cultured capsular bag at day 7 in experimental group II. PCNA positive cell (red arrow) was noted in control (A) (×200). PCNA positive cell was not noted in 1 µM (B), 10 µM (C) and 100 µM Cisplatin (D) (×100). |
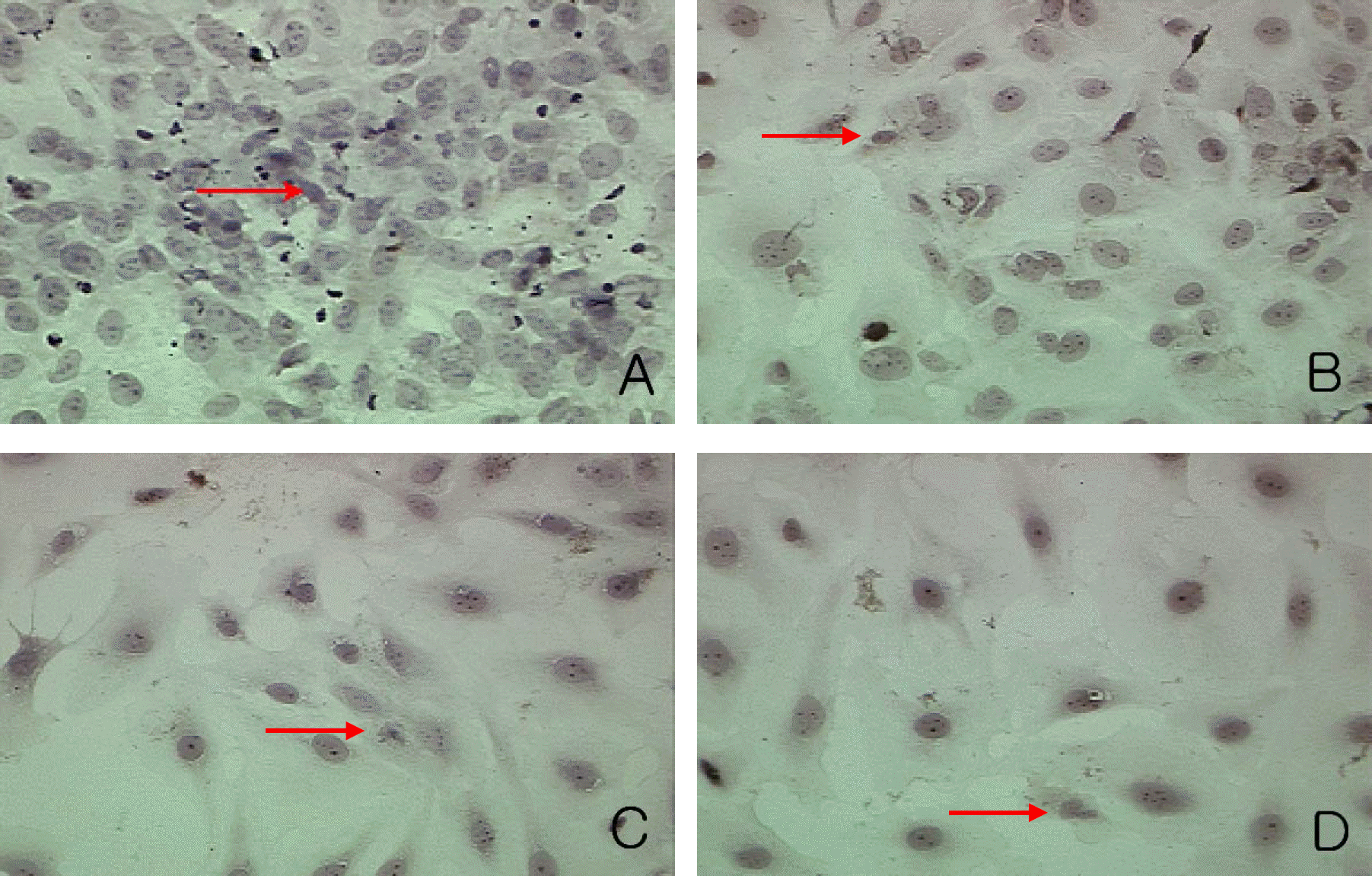 | Figure 11.TUNEL staining analysis in experimental group I. Magnification:400× Apoptotic body (red arrow) was seen in control group (A). The number of apoptotic bodies showed no significant change according to increasing concentrations of Paclitaxel (1 nM (B), 10 nM (C), 100 nM (D)). |
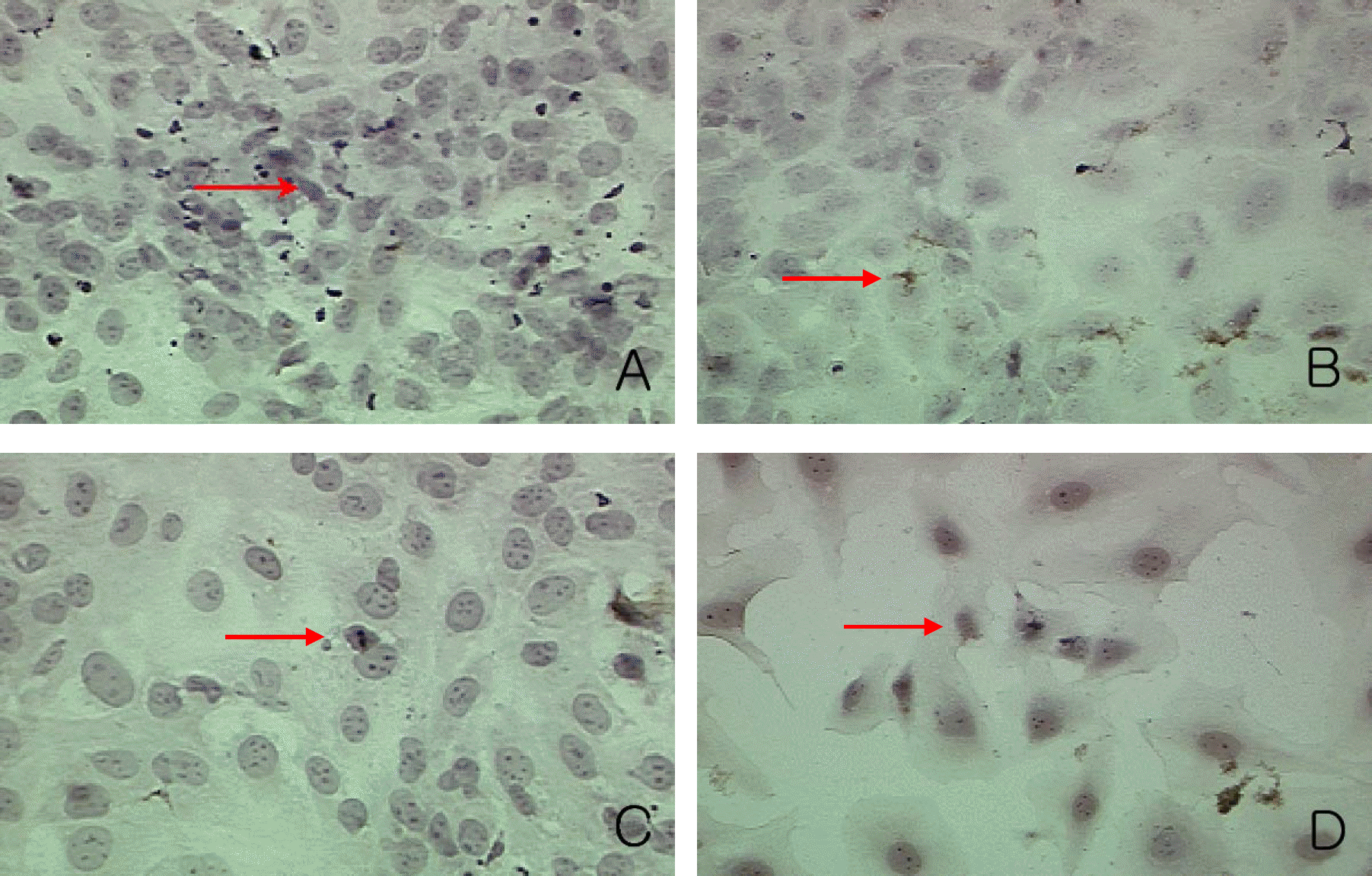 | Figure 12.TUNEL staining analysis in experimental group II. Magnification: 400× Apoptotic body (red arrow) was seen in control group (A). The number of apoptotic bodies showed no significant change according to increasing concentrations of Cisplatin (1 µM (B), 10 µM (C), 100 µM (D)). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download