Abstract
Purpose
To analyse the postoperative anatomical changes of maculae using optical coherent tomography after internal limiting membrane peeling during vitrectomy.
Methods
A retrospective review of 22 patients (22 eyes) who underwent vitrectomy with internal limiting membrane peeling was performed. Age, sex, fundus findings, BCVA, operation methods, complications, and postoperative OCT findings were recorded.
Results
Of 22 eyes, 12 eyes had epiretinal membranes, 5 eyes had macular holes, and 5 eyes had diffuse diabetic macular edemas. Eighteen of 22 eyes underwent internal limiting membrane peeling using ICG, with 1 eye treated with triamcinolone and 3 eyes treated with nothing. The postoperative improvement of BCVA was statistically significant in epiretinal membrane and diffuse macular edema patients (p<0.05), but insignificant in macular hole patients (p>0.05). Postoperative OCT findings of 17 eyes showed that they had returned to normal shape and thickness. However, among the 18 eyes that underwent ICG dye-assisted ILM, 3 eyes showed irregular macular thinning and 1 eye showed macular cystic changes. One eye treated with triamcinolone presented with a recurrent cytoid macular edema. No postoperative complications or recurrences were reported by epiretinal membrane patients.
Go to : 
References
1. Kim TH, Koh HJ, Kwon OW. Effect of removal of internal limiting membrane in macular hole surgery. J Korean Ophthalmol Soc. 1999; 40:1027–35.
2. Kim YS, Kim YB. Effect of internal limiting membrane peeling in indiopathic macular hole stage 3, 4. J Korean Ophthalmol Soc. 2001; 42:446–52.
3. Brooks HL. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000; 107:1939–48.

4. Gandorfer A, Messmer EM, Ulbig KW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000; 20:126–33.

5. Haritoglou C, Gass CA, Schaumberger M, et al. Long term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002; 134:661–6.
6. Fine BS. Limiting membranes of the sensory retina and pigment epithelium. An electron microscopic study. Arch Ophthalmol. 1961; 66:847–60.
7. Kowk AK, Li WWY, Pang CP, et al. Indocyanine green staining and removal of internal limiting membrane in macular hole surgery: histology and outcome. Am J Ophthalmol. 2001; 132:178–83.
8. Wolf S, Schnurbusch U, Wiedemann P, et al. Peeling of the basal membrane in the human retina. Ophthalmology. 2004; 111:238–43.

9. Uemoto R, Yamamoto S, Takeuchi S. Epimacular proliferative response following internal limiting membrane peeling for idiopathic macular holes. Graefes Arch Clin Exp Ophthalmol. 2004; 242:177–80.

10. Shah GK, Rosenblatt BJ, Blinder KJ. Triamcinolone-assisted internal limiting membrane peeling. Retina. 2005; 25:972–5.

11. Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peelig; pilot study. Ophthalmology. 2003; 110:62–4.
12. Sheidow TG, Blinder KJ, Holekamp N, et al. Outcome result in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003; 110:1697–701.
13. Mavrofrides E, Smiddy WE, Kitchens JW, et al. Indocyanine green-assisted internal limiting membrane peeling for macular holes. Toxicity? Retina. 2006; 26:637–44.

14. Haritoglou C, Gandorfer A, Gass C, et al. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003; 135:328–37.

15. Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2004; 242:995–9.

16. Uemura A, Kanda S, Sakamoto Y, Kita H. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003; 136:252–7.

17. Ferencz A, Somfai GM, Farkas A. Functional assessment of the possible toxicity of indocyanine green dye in macular hole surgery. Am J Ophthalmol. 2006; 142:765–70.

18. Lai TY, Kwok AK, Au AW, Lam DS. Assessment og macular function by multifocal electroretinography following epiretinal membrane surgery with indocyanine green-assisted internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol. 2007; 245:148–54.
19. Kim TW, Song SJ, Chung H, Yu HG. Internal limiting membrane peeling in surgical treatment of macular epiretinal membrane. J Korean Ophthalmol Soc. 2005; 46:989–94.
20. Ryan SJ, Schachat AP. Retina. 3rd ed.Vol. 2. Missouri: Mosby;2001. p. 977–8.
21. Uemoto R. Yamamoto S. Takeuchi S. Changes in retinal pigment epithelium after indocyanine green-assisted internal limiting lamina peeling during macular hole surgery. Am J Ophthalmol. 2005; 140:752–5.
22. Engelbrecht NE, Freeman J, Sternberg P, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002; 133:89–94.

23. Czajka MP, McCuen BW, Cummings TJ, et al. Effects of indocyanine green on the retina and retinal pigment epithelium in a porcine model of retinal hole. Retina. 2004; 24:275–82.

24. Gale JS, Prouls AA, Gonder JR, et al. Comparison of the in vitro toxicity of indocyanine green to that of trypan bleu in human retinal pigment epithelium cell cultures. Am J Ophthalmol. 2004; 138:64–9.
25. Maurico M, Julia HA, Dante PJ, et al. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina. 2004; 24:157–60.

26. Lee JE, Yoon TJ, Oum BS, et al. Toxicity of indocyanine green injected into the subretinal space: subretinal toxicity of indocyanine green. Retina. 2003; 23:675–81.
27. Biesen PR, Berenschot T, Vardaasdonk RM, et al. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000; 84:1372–5.
28. Gandorfer A, Haritoglou G, Kampik A, Kampik A. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003; 44:316–23.

29. Rizzo S, Belting C, Genovesi-Ebert F, et al. Modified technique for safer indocyanine-green-assisted peeling of the internal limiting membrane during vitrectomy for macular hole repair. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1615–9.

30. Tognetto D, Haritoglou C. Kampik A, Ravalico G. Macular ededma and visual loss after macular pucker surgery with ICG-assisted internal limiting membrane peeling. Eur J Ophthalmol. 2005; 15:289–91.
31. Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal liminting membrane in macular hole surgery. Am J Ophthalmol. 2001; 132:363–8.
32. Ryan SJ, Schachat AP. Retina. 3rd ed.Vol. 2. Missouri: Mosby;2001. p. 967–8.
Go to : 
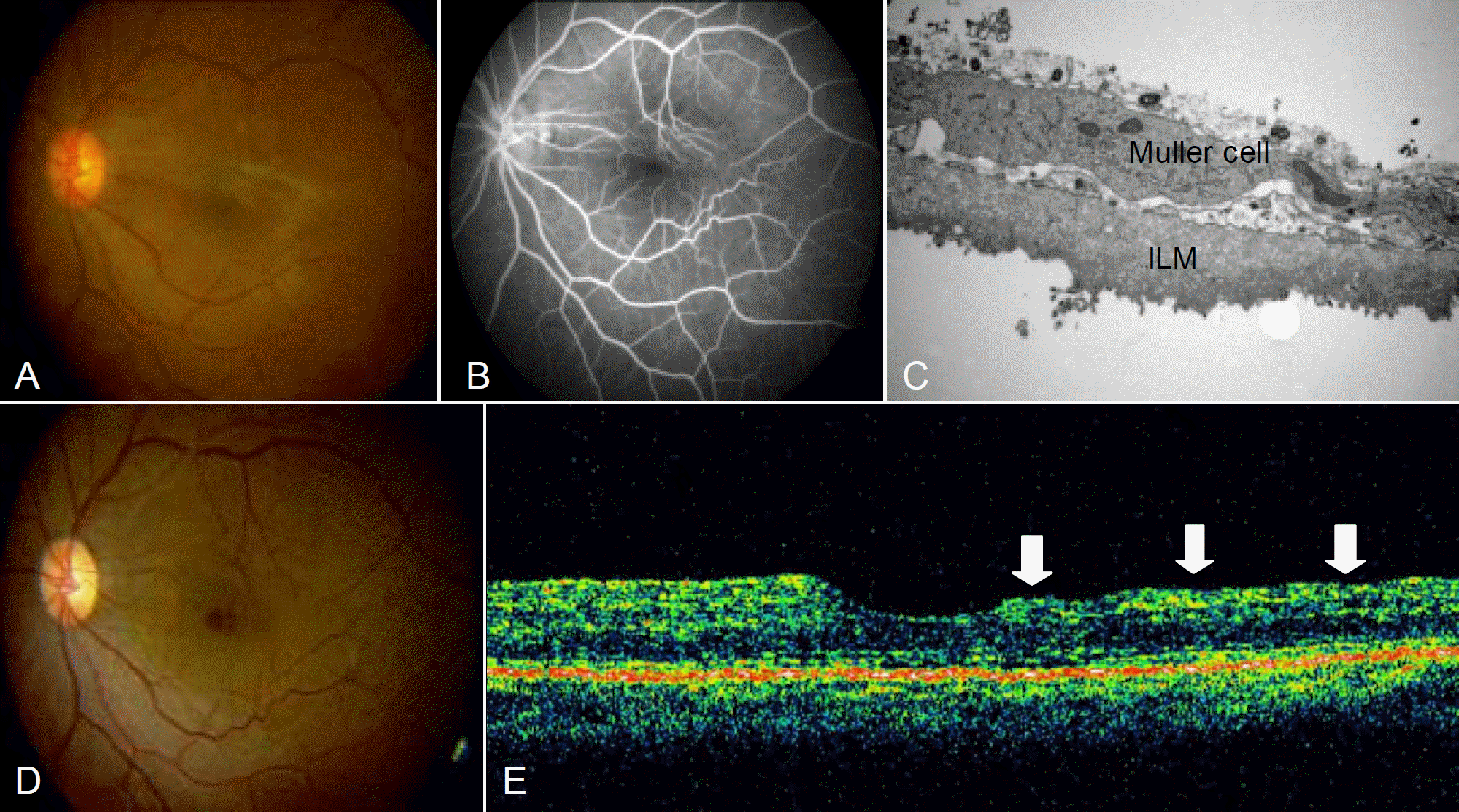 | Figure 1.(A) preoperative fundus photography; (B) preoperative fluorescien angiography; (C) peeled ILM, electromicroscopic finding X 150,000; (D) fundus photography at 1 year after operation; (E) OCT finding shows irregular surface and thinning of the macular area. |
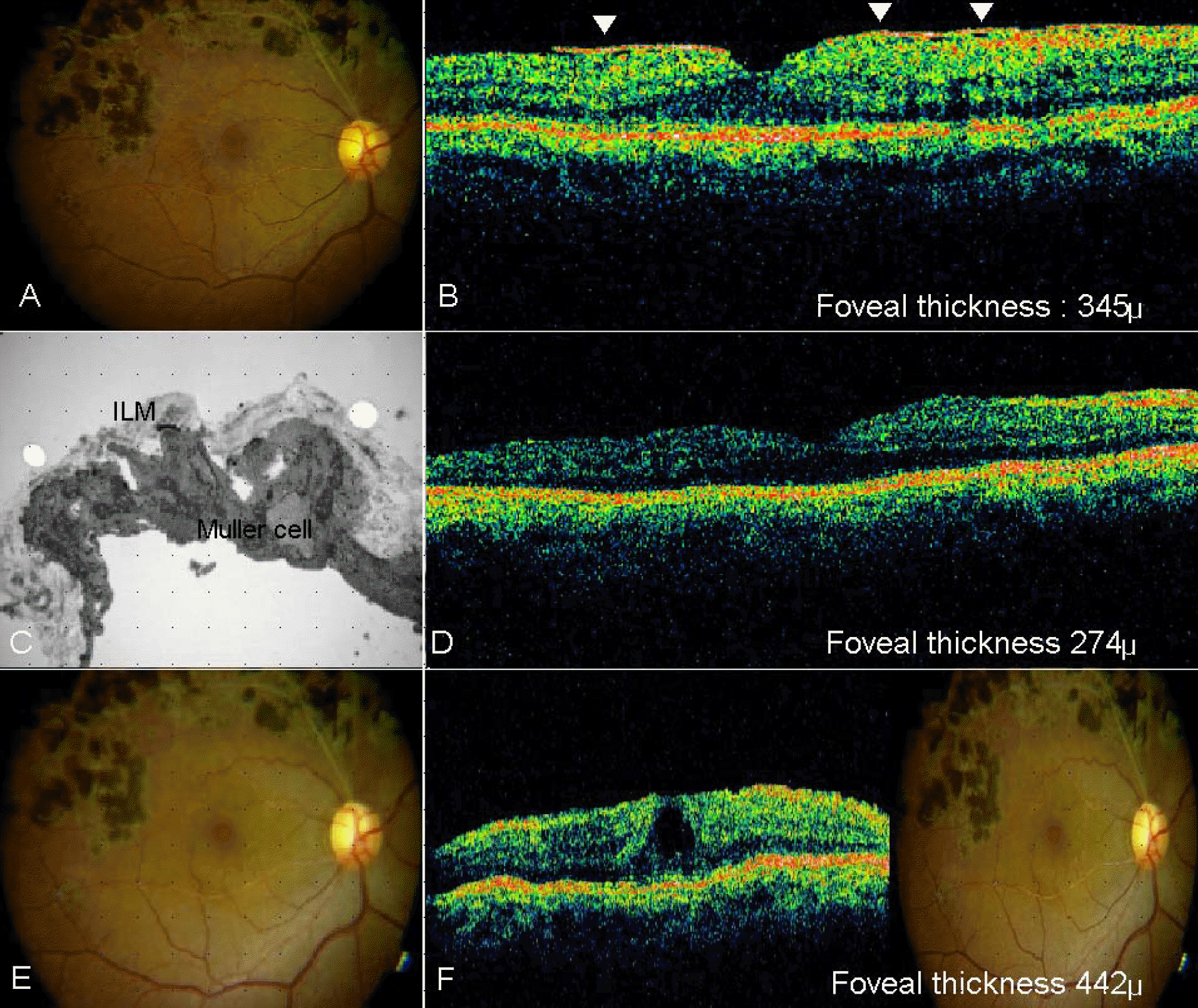 | Figure 2.(A) preoperative fundus photography; (B) preoperative OCT finding; (C) peeled ILM, electromicroscopic finding X 20,000; (D) OCT finding at 1 day after operation; (E) fundus photograph at 6 month after operation; (F) OCT finding at 6 month after operation. |
Table 1.
Demographics of 5 patients with postoperative abnormal OCT change
Table 2.
Comparison of log MAR(minimum angle of correction) best corrected visual acuity measured preoperatively and postopertively in each disease
| Group | Preoperative BCVA | Postoperative BCVA | * p-value |
|---|---|---|---|
| ERM (n=12) | 0.37 | 0.14 | 0.01 |
| MH (n=5) | 0.99 | 0.67 | 0.22 |
| DME (n=5) | 0.68 | 0.44 | 0.04 |
Table 3.
Comparison of postoperative BCVA between patients with normal and abnormal OCT findings
|
OCT finding |
* p-value | |
|---|---|---|
| Normal | Abnormal | |
| 0.36 | 0.22 | 0.59 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download