Abstract
Purpose
To compare the efficacy and safety of fibrin glue and sutures for amniotic membrane transplantation after wide excision of primary pterygium.
Methods
Forty-three eyes of 37 patients underwent amniotic membrane transplantation for primary pterygium with a minimum follow-up period of six months. Twenty-three eyes of 18 patients underwent surgery with fibrin glue and 20 eyes of 19 patients underwent surgery with sutures. Recurrence rates, complications, operating time were evaluated.
Results
With a minimum of six-month of follow-up, fibrovascular tissue in the excised area, not invading the cornea (conjunctival recurrence), was noted in four eyes (17.4%) and fibrovascular tissue invading the cornea (corneal recurrence) was noted in two eyes (8.7%) in the fibrin glue group. Conjunctival recurrence was noted in five eyes (25%) and corneal recurrence was noted in one eye (5%) in the suture group. There was no significant difference in the recurrence rates between the two groups. Mean operating time in the fibrin glue group (25.2±3.5 minutes) was significantly shorter than in the suture group (40.5±3.6 minutes) (p=0.001, Students t-test). Complications included sub-amniotic membrane hemorrhage in three eyes (13%), and granuloma in one eye (4.3%) in the fibrin glue group, sub-amniotic membrane hemorrhage in four eyes (20%), granuloma in three eyes (15%), and wound dehiscence in one eye (5%) in the suture group.
References
1. Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001; 119:695–706.
2. Sakoonwatanyoo P, Tan DT, Smith DR. Expression of p63 in pterygium and normal conjunctiva. Cornea. 2004; 23:67–70.

3. Solomon A, Grueterich M, Li DQ, et al. Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 2003; 44:573–80.

4. Di Girolamo N, McCluskey P, Lloyd A, et al. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000 Mar; 41:671–9.
5. Li DQ, Lee SB, Gunja-Smith Z, Liu Y, et al. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001; 119:71–80.
6. Dushku N, Hatcher SL, Albert DM, Reid TW. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch Ophthalmol. 1999; 117:1593–9.

7. Threlfall TJ, English DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999; 128:280–7.

8. Tsai YY, Cheng YW, Lee H, et al. Oxidative DNA damage in pterygium. Mol Vis. 2005; 11:71–5.
9. Dadeya S, Malik KP, Gullian BP. Pterygium surgery: conjunctival rotation autograft versus conjunctival autograft. Ophthalmic Surg Lasers. 2002; 33:269–74.

10. Tan DT, Chee SP, Dear KB, et al. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997; 115:1235–40.

11. Al Fayez MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002; 109:1752–5.

12. Gris O, Guell JL, del Campo Z. Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology. 2000; 107:270–3.

13. Shimazaki J, Yang HY, Tsubota K. Limbal autograft transplantation for recurrent and advanced pterygia. Ophthalmic Surg Lasers. 1996; 27:917–23.

14. Ti SE, Tseng SC. Management of primary and recurrent pterygium using amniotic membrane transplantation. Curr Opin Ophthalmol. 2002; 13:204–12.

15. Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001; 108:449–60.

16. Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005; 24:141–50.

17. Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997; 104:974–85.

18. Jurgenliemk-Schulz IM, Hartman LJ, Roesink JM, et al. Prevention of pterygium recurrence by postoperative single-dose beta-irradiation: a prospective randomized clinical double-blind trial. Int J Radiat Oncol Biol Phys. 2004; 59:1138–47.
19. Liddy BS, Morgan JF. Triethylene thiophosphoramide (thio-tepa) and pterygium. Am J Ophthalmol. 1966; 61:888–90.
20. Segev F, Jaeger-Roshu S, Gefen-Carmi N, Assia EI. Combined mitomycin C application and free flap conjunctival autograft in pterygium surgery. Cornea. 2003; 22:598–603.

21. Sharma A, Gupta A, Ram J, Gupta A. Low-dose intraoperative mitomycin-C versus conjunctival autograft in primary pterygium surgery: long term follow-up. Ophthalmic Surg Lasers. 2000; 31:301–7.

22. Tananuvat N, Martin T. The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea. 2004; 23:458–63.

23. Koranyi G, Seregard S, Kopp ED. The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 2005; 83:298–301.

24. Uy HS, Reyes JM, Flores JD, Lim-Bon-Siong R. Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology. 2005; 112:667–71.

25. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004; 88:911–4.

26. Marticorena J, Rodriguez-Ares MT, Tourino R, et al. Pterygium surgery: conjunctival autograft using a fibrin adhesive. Cornea. 2006; 25:34–6.

27. Hick S, Demers PE, Brunette I, et al. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005; 24:369–77.
28. Sharma A, Kaur R, Kumar S, et al. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003; 110:291–8.

29. Pfister RR, Sommers CI. Fibrin sealant in corneal stem cell transplantation. Cornea. 2005; 24:593–8.

30. Velazquez AJ, Carnahan MA, Kristinsson J, et al. New dendritic adhesives for sutureless ophthalmic surgical procedures: in vitro studies of corneal laceration repair. Arch Ophthalmol. 2004; 122:867–70.
31. Refojo MF, Dohlman CH, Ahmad B, et al. Evaluation of adhesives for corneal surgery. Arch Ophthalmol. 1968; 80:645–56.

32. Webster RG Jr, Slansky HH, Refojo MF, et al. The use of adhesive for the closure of corneal perforations: report of two cases. Arch Ophthalmol. 1968; 80:705–9.
33. Cohen RA, McDonald MB. Fixation of conjunctival autografts with an organic tissue adhesive. Arch Ophthalmol. 1993; 111:1167–8.

34. Yoon KC, Heo H, Jeong IY, et al. The use of fibrin glue for conjunctival autotransplantation in pterygium. J Korean Ophthalmol Soc. 2006; 47:198–204.
35. Paek KU, Park HS, Kim YI. Ocular surface reconstruction with amniotic membrane transplantation in pterygium. J Korean Ophthalmol Soc. 1999; 40:1178–83.
36. Jang JH, Choi TH. The effect of amniotic membrane transplantation for pterygium excision. J Korean Ophthalmol Soc. 2005; 46:597–604.
Figure 1.
Classification of pterygium. (A) Grade T1 (atrophic) episcleral vessels are unobscured. (B) Grade T2 (intermediate) episcleral vessels are partially obscured. (C) Grade T3 (fleshy) episcleral vessels are totally obscured.
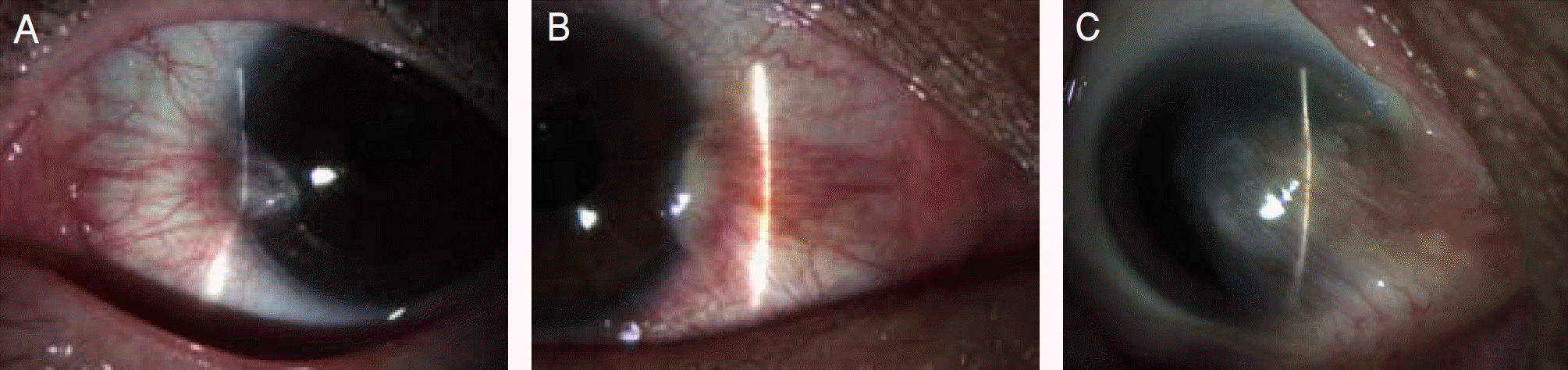
Figure 2.
Grading of recurrence after pterygium surgery. (A) Grade 0, normal appearance of the operated site. (B) Grade 1, fine episcleral vessels in the excised area. (C) Grade 2, fibrovascular tissue in the excised area, reaching to the limbus, but not invading the cornea (conjunctival recurrence). (D) Grade 3, fibrovascular tissue invading the cornea (corneal recurrence).
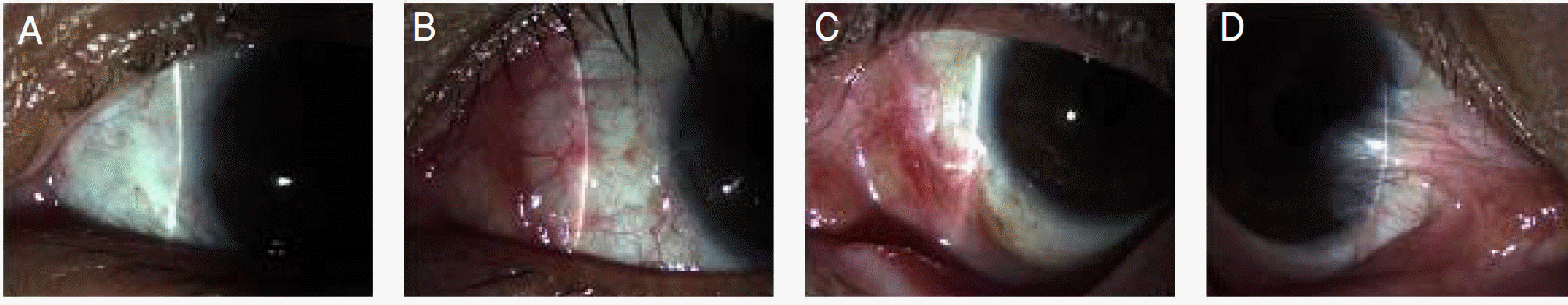
Figure 3.
Procedure to perform amniotic membrane transplantation using fibrin glue after wide excision of pterygium. (A) 1:1,000 epinephrin was dropped. (B) episcleral traction sutures were placed. (C) excision and blunt dissection of pterygium from the cornea and sclera by using conjunctival scissors. (D) remnant pterygium of the cornea was dissected off by corneal forceps.(E) wide excision of the subconjunctival Tenon's tissue was done by the Ellman cautery. (F) amniotic membrane is peeled off the carrier paper and spread over the cornea. (G) a drop of thrombin solution on bare sclera. (H) a dorp of fibrinogen solution on the amniotic membrane. (I) the amniotic membrane was then immediately transferred onto the bare sclera. (J) smoothering of the amniotic membrane was done by a muscle hook. (K) the amniotic membrane was sutured to the episclera and conjuctiva to facilitate regeneration of the conjunctiva over the amniotic membrane. (L) triamcinolon was injected.
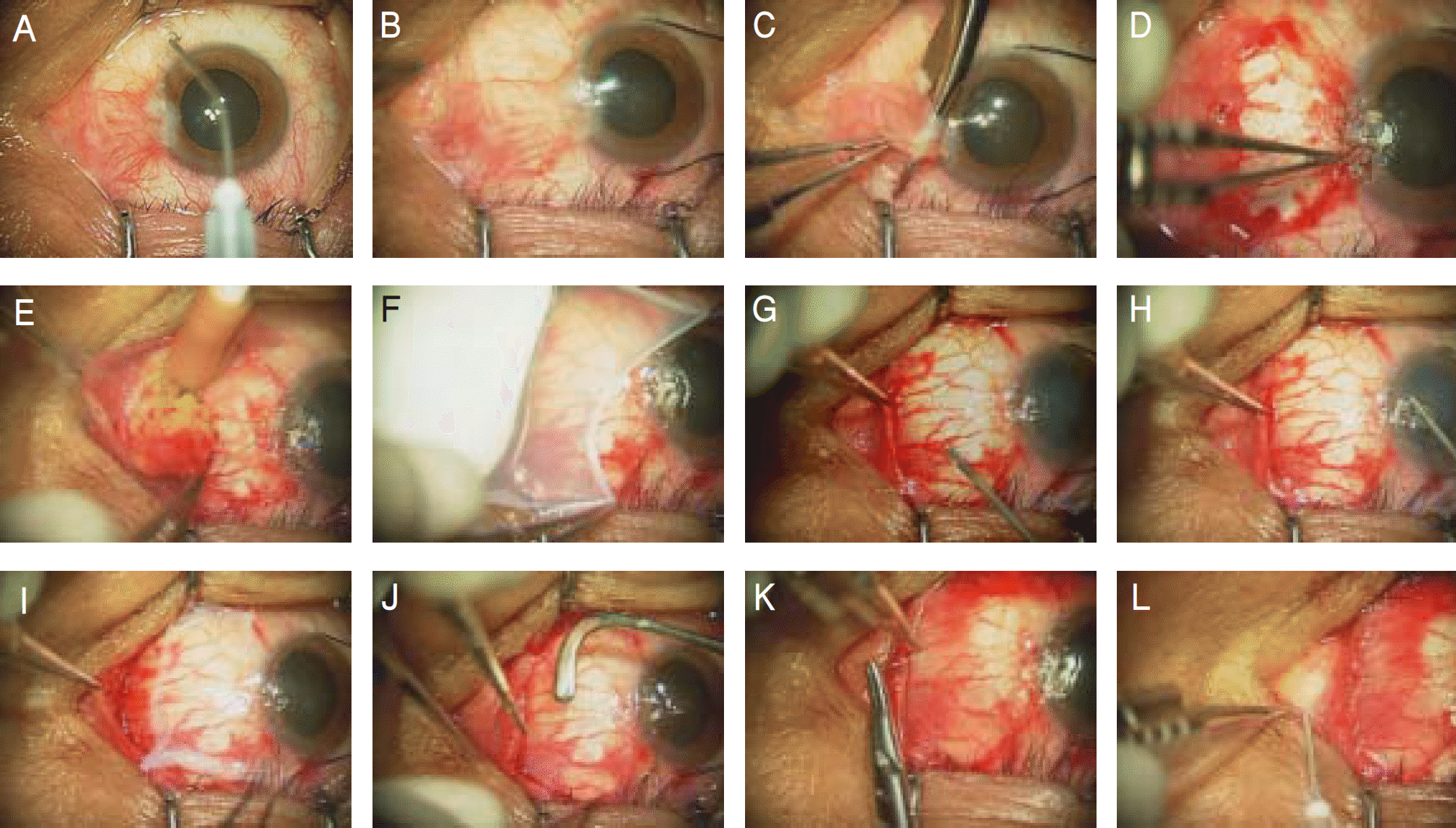
Figure 4.
(A) The right eye before surgery. There is a large and fleshy primary pterygium occluding the visual axis. (B-C) At 1 day after surgery. The amniotic membrane area is transparent and nonvascularized B. Note transplanted amniotic membrane stained by fluorescein C. (D-E) At 3 weeks after surgery. The surgical wound is healed D and complete epithelization is done under fluorescein staining E. (F) At 6 months after surgery. There is a smooth and quiet conjunctival surface at the operated site.

Figure 5.
Postoperative complications. (A) sub-amniotic membrane hemorrhage. (B) granuloma at the operated site.
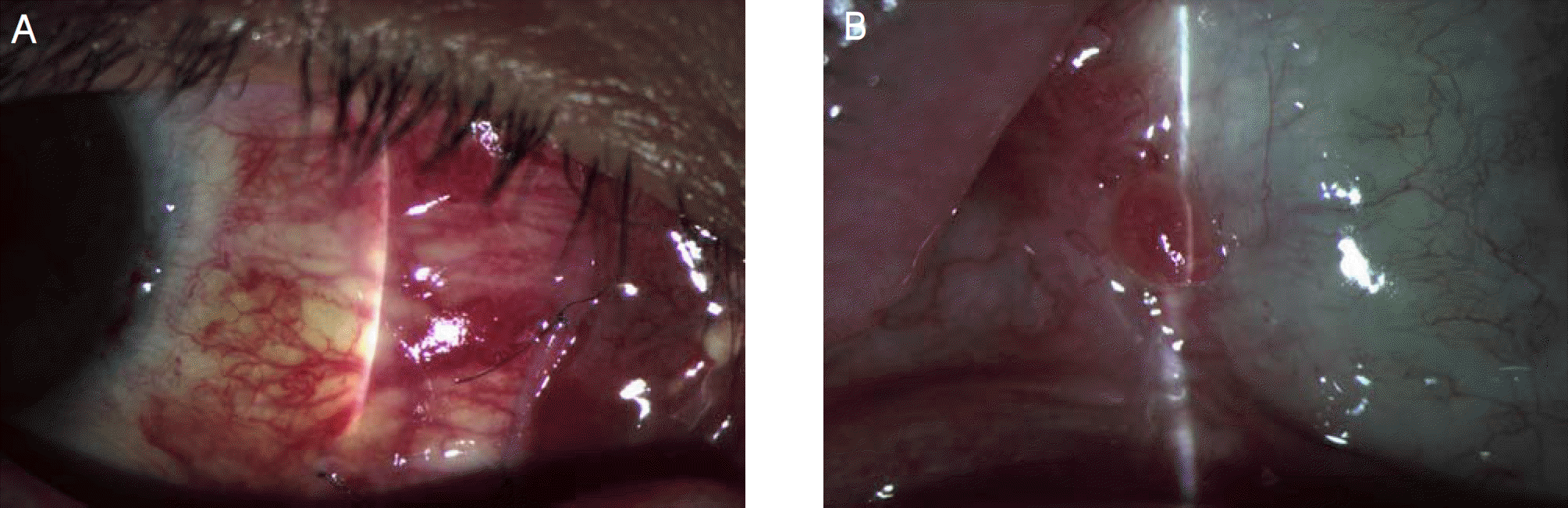
Table 1.
Demographic datas between fibrin glue group and suture group
| Fibrin glue (23 eyes) | Suture (20 eyes) | p-value | |
|---|---|---|---|
| Age (years) (Mean±SD*) | 60.5±11.7 | 57.1±13.1 | 0.41† |
| Sex (Male: Female) | 10:8 | 6:13 | 0.14‡ |
| Follow-up (weeks) (Mean±SD*) | 28.4±2.8 | 26.9±2.6 | 0.10† |
| Grade T2: Grade T3 (eyes) | 9:14 | 7:13 |
Table 2.
Comparison of recurrence rates between fibrin glue group and suture group
|
Fibrin glue |
Suture |
|||
|---|---|---|---|---|
| Grade T2 | Grade T3 | Grade T2 | Trade T3 | |
| G2 (conjunctival recurrence) | 0 | 4 eyes | 1 eye | 4 eyes |
| G3 (corneal recurrence) | 0 | 2 eyes | 0 | 1 eye |
| Recurrence rates | ||||
| Between T2 and T3 | 0/9 (0%)* | 6/14 (42.9%)* | 1/7 (14.3%)† | 5/13 (38.5%)† |
| Total | 6/23 (26.1%)‡ | 6/20 (30%)‡ | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download