Abstract
Purpose
To evaluate the inhibitory effect of tranilast on the formation of posterior capsular opacity (PCO) after a cataract operation ex vivo and in a rabbit model.
Methods
A human lens epithelial cell line (B3) was treated with 0.005-0.1 mM tranilast. Cytotoxicity assessment and effective dosage determination of tranilast were performed using MTT assays. B3 cell lines were cultured in Eagle's minimal essential medium (EMEM) containing 20% FBS with different concentrationsof tranilast, and morphological differences were observed. To investigate the effect of tranilast on cytokine production in B3 cell lines, cells were treated with 0.01 mM tranilast and expression profiles of cytokines were analyzed by RT-PCR. After performing phacoemulsification and intraocular lens implantation in 10 white rabbits, 0.5% tranilast eye drops were given 4 times per day, and the severity of PCO was evaluated bi-weekly using POCOman for 8 weeks after the operation.
Results
Cell death was observed in the 0.05 mM tranilast-treated B3 cell lines, and inhibition of epithelial- mesenchymal transition (EMT) was also observed in the 0.01 mM tranilast-treated B3 cell lines. TGF-β1/2, IL-18, and CDK7 mRNA expression decreased in the 0.01 mM tranilast-treated B3 cell lines. Significant suppression of PCO formation was observed in rabbits treated with 0.5% tranilast eye drops 5 weeks post operative ( p<0.05).
Go to : 
References
1. Apple DJ, Solomon KD, Tetz MR. . Posterior capsule opacification. Surv Ophthalmol. 1992; 37:73–116.

2. Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998; 105:1213–21.

3. Sundelin K, Sjöstrand J. Posterior capsule opacification 5 years after extracapsular cataract extraction. J Cataract Refract Surg. 1999; 25:246–50.
4. Linnola RJ, Werner L, Pandey SK. . Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. Part 2: explanted intraocular lenses. J Cataract Refract Surg. 2000; 26:1807–18.
5. Maloof A, Neilson G, Milverton EJ. Selective and specific targeting of lens epithelial cells during cataract surgery using sealed- capsule irrigation. J Cataract Refract Surg. 2003; 29:1566–8.

6. Peng Q, Visessook N, Apple DJ. . Surgical prevention of posterior capsule opacification. Part 3: Intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg. 2000; 26:198–213.
7. Ravalico G, Tognetto D, Palomba M. . Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg. 1996; 22:98–103.

8. Apple DJ. Influence of intraocular lens material and design on postoperative intracapsular cellular reactivity. Trans Am Ophthalmol Soc. 2000; 98:257–83.
9. Trocme SD, Li H. Effect of heparin-surface-modified intraocular lenses on postoperative inflammation after phacoemulsification: a randomized trial in a United States patient population. Heparin-Surface-Modified Lens Study Group. Ophthalmology. 2000; 107:1031–7.
10. Gatinel D, Lebrun T, Le Toumelin P, Chaine G. Aqueous flare induced by heparin-surface-modified poly(methyl methacrylate) and acrylic lenses implanted through the same-size incision in patients with diabetes. J Cataract Refract Surg. 2001; 27:855–60.

11. Nishi O, Nishi K. Effect of the optic size of a single-piece acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg. 2003; 29:348–53.

12. Nishi O, Nishi K, Mano C. . The inhibition of lens epithelial cell migration by a discontinuous capsular bend created by a band-shaped circular loop or a capsule-bending ring. Ophthalmic Surg Lasers. 1998; 29:119–25.
13. Brabletz T, Hlubek F, Spaderna S. . Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005; 179:56–65.
14. Kim IY, Kim MM, Kim SJ. Transforming growth factor-beta : biology and clinical relevance. J Biochem Mol Biol. 2005; 38:1–8.
15. Wang M, Zhang JJ, Jackson TL. . Safety and efficacy of intracapsular tranilast microspheres in experimental posterior capsule opacification. J Cataract Refract Surg. 2007; 33:2122–8.

16. Platten M, Ho PP, Youssef S. . Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005; 310:850–5.

17. Lee JS, Shin YG, Jung JH, Oum BS. The effects of tranilast(R) on the cellular proliferation, collagen synthesis, and secretion of TGF-beta1 in bovine retinal pigment epithelial cells. J Korean Ophthalmol Soc. 2003; 44:1205–11.
18. Lee JS, Jung SC. The Effects of Tranilast gamma on the Cellular Proliferation and alpha-Smooth Muscle Actin Expression in the Cultured Keratocytes of Rabbit. J Korean Ophthalmol Soc. 2004; 45:668–74.
19. Lee JE, Han HJ, Lee JS, Oum BS. Effect of Tranilast on the Proliferation of Human Corneal Keratocytes in Vitro. J Korean Ophthalmol Soc. 2005; 46:510–20.
20. Song JS, Jung HR, Kim HM. Effects of topical tranilast on corneal haze after photorefractive keratectomy. J Cataract Refract Surg. 2005; 31:1065–73.

21. Bender L, Spalton DJ, Uyanonvara B. . POCOman: new system for quantifying posterior capsule opacification. J Cataract Refract Surg. 2004; 30:2058–63.
22. Spiecker M, Lorenz I, Marx N, Darius H. Tranilast inhibits cytokine-induced nuclear factor kappaB activation in vascular endothelial cells. Mol Pharmacol. 2002; 62:856–63.
23. Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. Jpn J Pharmacol. 1992; 60:91–6.
24. Isaji M, Aruga N, Naito J, Miyata H. Inhibition by tranilast of collagen accumulation in hypersensitive granulomatous inflammation in vivo and of morphological changes and functions of fibroblasts in vitro. Life Sci. 1994; 55:287–92.
25. Isaji M, Miyata H, Ajisawa Y, Yoshimura N. Inhibition by tranilast of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF)-induced increase in vascular permeability in rats. Life Sci. 1998; 63:71–4.

26. Charng MJ, Wu CH. Transcriptional activation of p21 by Tranilast is mediated via transforming growth factor beta signal pathway. Br J Pharmacol. 2006; 147:117–24.
27. Kanada T, Shikita M, Tsuneoka K. . Effect of macrophage colony-stimulating factor on the function of resident peritoneal macrophages. J Pharmacobiodyn. 1987; 10:215–9.

28. Shin D, Minn KW. The effect of myofibroblast on contracture of hypertrophic scar. Plast Reconstr Surg. 2004; 113:633–40.

29. Inglis JJ, Criado G, Andrews M. . The anti-allergic drug, N-(3',4'-dimethoxycinnamonyl) anthranilic acid, exhibits potent anti-inflammatory and analgesic properties in arthritis. Rheumatology (Oxford). 2007; 46:1428–32.

30. Cooper K, Young J, Wadsworth S. . Reduction of post-surgical adhesion formation with tranilast. J Surg Res. 2007; 141:153–61.

31. Ito S, Sakamoto T, Tahara Y. . The effect of tranilast on experimental proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1999; 237:691–6.

32. Chihara E, Dong J, Ochiai H, Hamada S. Effects of tranilast on filtering blebs: a pilot study. J Glaucoma. J Glaucoma. 2002; 11:127–33.
33. Wallenfang MR, Seydoux G. cdk-7 is required for mRNA transcription and cellcycle progression in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2002; 99:5527–32.
34. Bochar DA, Pan ZQ, Knights R. . Inhibition of transcription by the trimeric cyclin-dependent kinase 7 complex. J Biol Chem. 1999; 274:13162–6.

35. Okamura H, Tsutsui H, Komatsu T. . Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995; 378:88–91.
36. Burbach GJ, Naik SM, Harten JB. . Interleukin-18 expression and modulation in human corneal epithelial cells. Curr Eye Res. 2001; 23:64–8.

37. Jiang HR, Wei X, Niedbala W. . IL-18 not required for IRBP peptide-induced EAU: studies in gene-deficient mice. Invest Ophthalmol Vis Sci. 2001; 42:177–82.
38. Nagai N, Ito Y, Okamura H. Involvement of interleukin 18 in cataract development in hereditary cataract UPL rats. J Biochem. 2007; 42:597–603.

39. Tobari I, Iwaki Y, Miyake K. Effect of tranilast eyedrops in preventing posterior capsule opacification. J Cataract Refract Surg. 1999; 25:1394–9.
40. Miyazawa Y, Iwaki Y, Yataka M, Tobari I. Effect of different concentrations of tranilast on posterior capsule opacification. Nippon Ganka Gakkai Zasshi. 2001; 105:442–6.

41. Wang M, Zhang JJ, Jackson TL. . Safety and efficacy of intracapsular tranilast microspheres in experimental posterior capsule opacification. J Cataract Refract Surg. 2007; 33:2122–8.

42. Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000; 41:961–4.
Go to : 
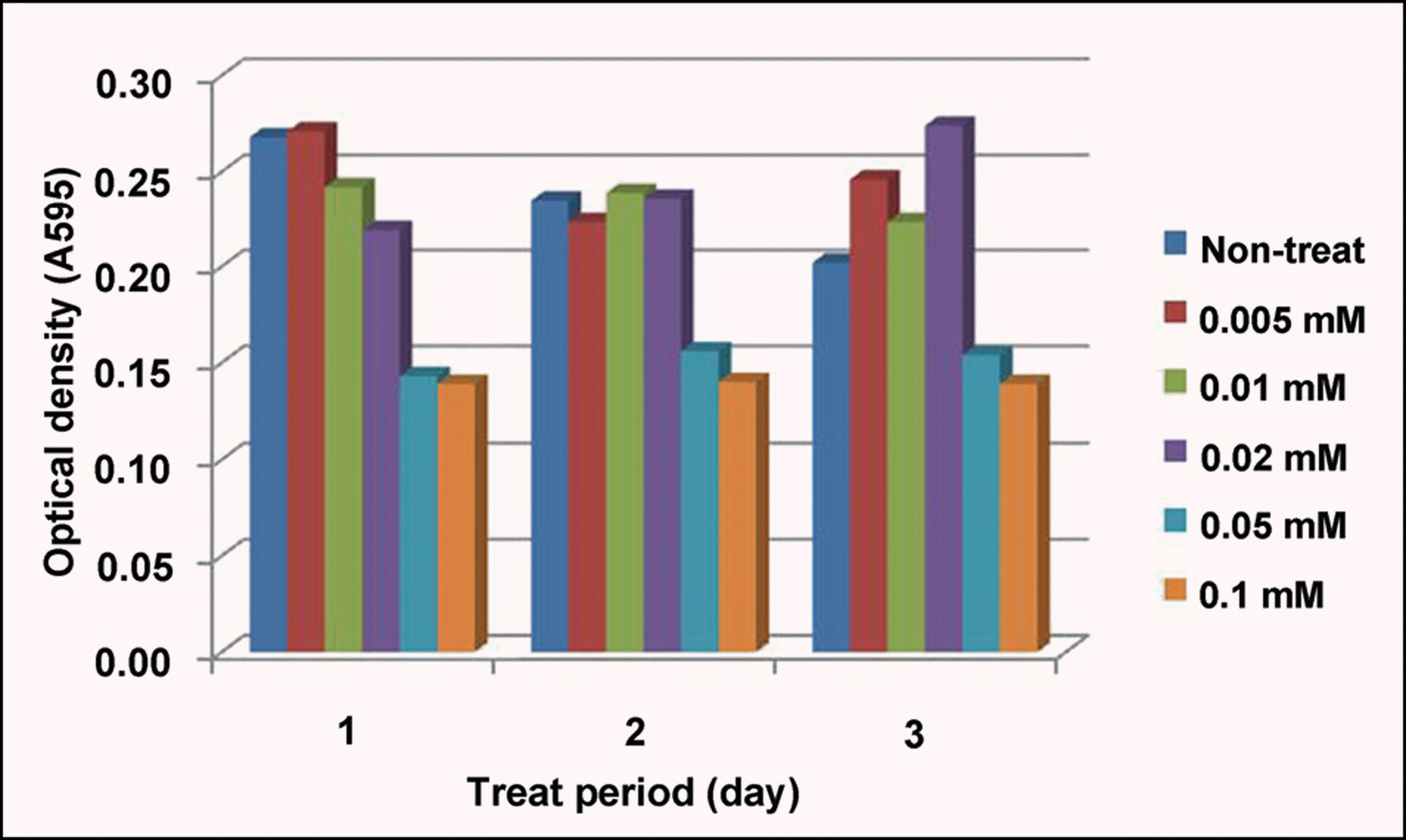 | Figure 1.The cytotoxicity of tranilast in B3 cell lines. The rapid decrease of proliferation was found at above 0.05 mM of tranilast, which was constant regardless of the cultivation time. |
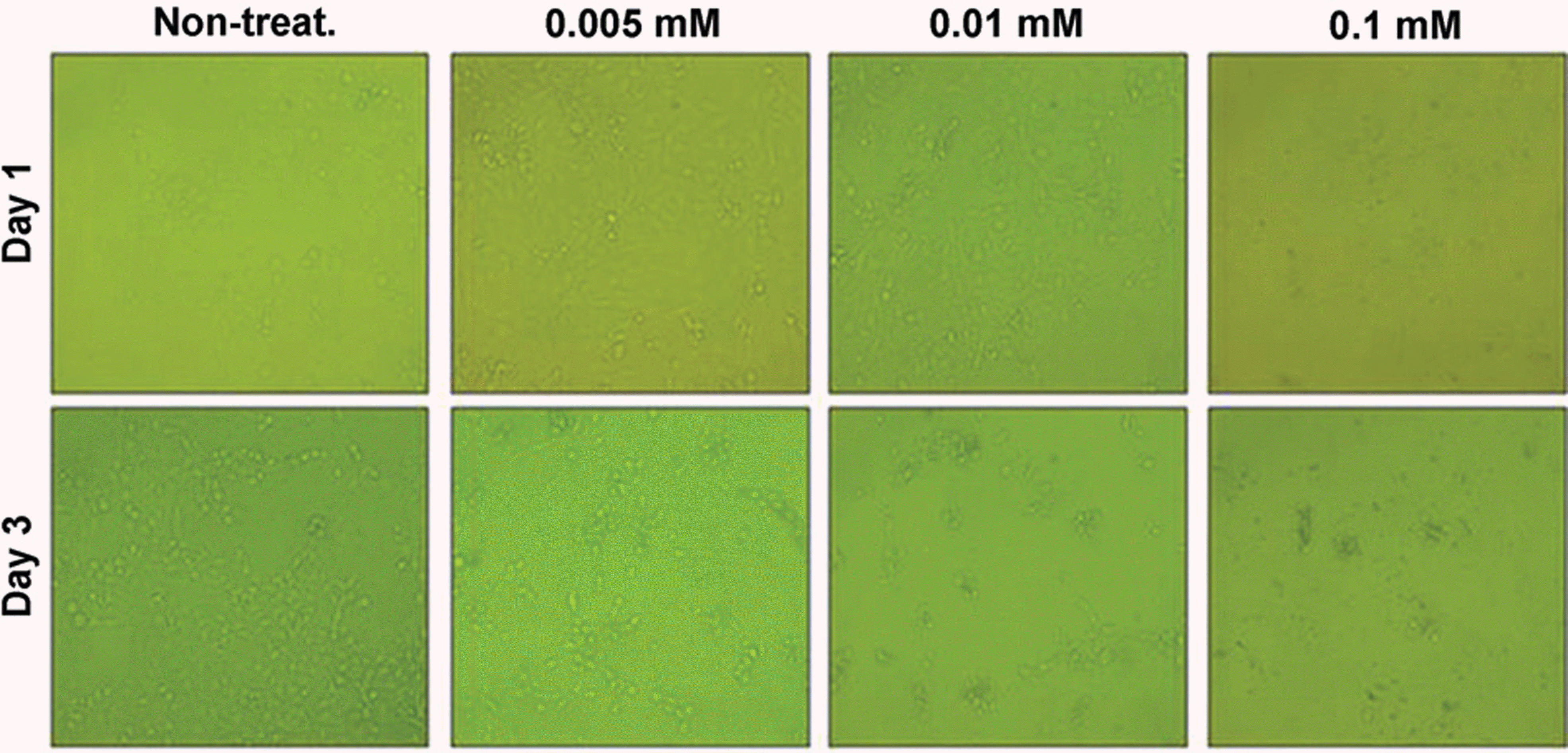 | Figure 2.Phase-contrast microscopic observation of 0.005-0.1 mM tranilast-treated B3 cell lines. Although non-treated-B3 cells showed spindle-shaped fibroblastic morphology, 0.01 mM tranilast-treated cells did not showed any morphological changes. 0.005 mM tranilast-treated cells showed slightly inhibited proliferation and morphological changes similar to non-treated cells. 0.1 mM tranilast-treated cells were detached and dead even in 1 hour after treatment. |
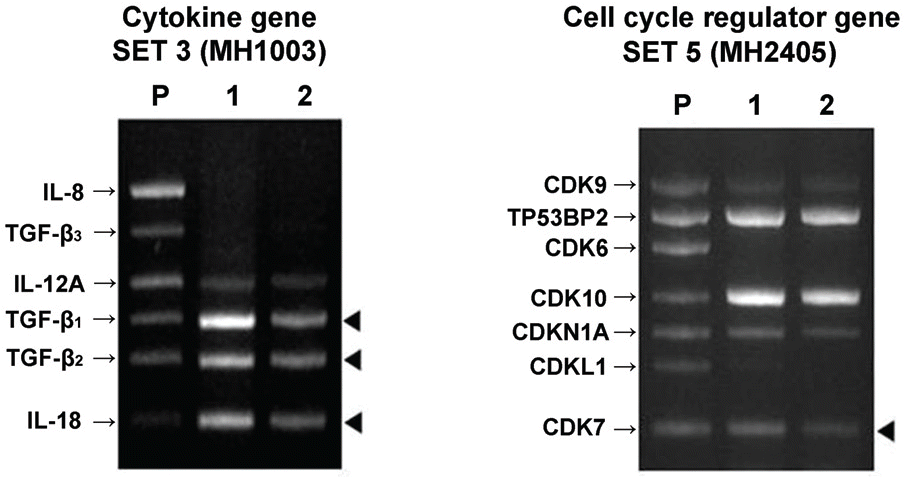 | Figure 3.Messenger RNA expression of cytokines in B3 cell lines; P=positive control 1=control group with DMSO2=0.01 mM tranilast-treatedgroup. The black arrowheads denote the prominent decrease in comparison with the control. |
 | Figure 4.PCO severity grading with POCOman software. Retroilluminated picture A was transformed into a black-and-white picture B. The margin of anterior capsule should be marked then the grid is allocated automatically. Using the standard photography (grade 1-3), the severity score was calculated with the formula as follows: Severity score=(3×Red (grade3)+ 2×Yellow (grade2)+1×Blue (grade1)) / total area. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download