Abstract
Purpose
We performed quantitative analysis of aqueous flare to evaluate the influence of diabetes mellitus on the flare values reflecting microvascular injuries of the retina.
Methods
We compared aqueous flare values of diabetic patients without diabetic retinopathy (80 patients, 160 eyes), with those of normal control group (21 persons, 42 eyes) and diabetic retinopathy patients (20 patients, 40 eyes). The correlation between flare values and the risk factors of diabetic retinopathy was evaluated in diabetic patients without diabetic retinopathy.
Go to : 
References
1. Jung H. Treatment of systemic disease: Diabetic Mellitus and Eye. Seoul: Seoul National University Edit co;2000. p. 145–8.
2. Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy.Early Treatment Diabetic Retinopathy Study Report No 9. Ophthalmology. 1991; 98:766–85.
3. Ino-ue M, Azumi A, Shirabe H. . Laser flare in diabetics: correlation with retinopathy and aqueous protein concentration. Br J Ophthalmol. 1995; 79:299–300.
4. Blum M, Muller UA, Höche A. . Flare measurement and albuminuria in type I diabetics. Klin Monatsbl Augenheilkd. 1998; 212:80–3.
5. Oshika T, Kato S, Funatsu H. Quantitative assessment of aqueous flare intensity in diabetes. Graefes Arch Clin Exp Ophthalmol. 1989; 227:518–20.

6. Tost F, Heilmann P, Lautenschlager C. Aqueous flare measurement with a laser flare cellmeter in eyes with diabetic retinopathy. Ophthalmologica. 1995; 209:56–9.

7. Zaczek A, Hallas K, Zetterstrom C. Aqueous flare intensity in relation to different stages of diabetic retinopathy. Eur J Ophthalmol. 1999; 9:158–64.

8. Nguyen NX, Schonherr U, Kuchle M. Aqueous flare and retinal capillary changes in eyes with diabetic retinopathy. Ophthalmologica. 1995; 209:145–8.

9. Moriarty AP, Spalton DJ, Moriarty BJ. . Studies of the blood-aqueous barrier in diabetes mellitus. Am J Ophthalmol. 1994; 117:768–71.

10. Sawa M, Tsurimaki Y, Tsuru T, Shimizu Hl. New quantitative method to determine protein concentration and cell number in the aqueous in vivo. Jpn J Ophthalmol. 1988; 32:132–42.
11. Gonzales CA, Ladas JG, Davis JL. . Relationships between laser flare photometry values and complications of uveitis. Arch Ophthalmol. 2001; 119:1763–9.

12. Ladas JG, Yu F, Loo R. . Relationship between aqueous humor protein level and outflow facility in patients with uveitis. Invest Ophthalmol Vis Sci. 1992; 33:2878–84.
13. Guex-Crosier Y, Pittet N, Herbort CP. Sensitivity of laser flare photometry to monitor inflammation in uveitis of the posterior segment. Ophthalmology. 1995; 102:613–21.

14. Guex-Crosier Y, Pittet N, Herbort CP. Sensitivity of laser flare photometry in the appraisal and management of intraocular inflammation in uveitis. Ophthalmology. 1994; 101:728–35.
15. Shah SM, Spalton DJ, Tayor JC. Correlation between laser flare measurements and anterior chamber protein concentrations. Invest Ophthalmol Vis Sci. 1992; 33:2878–84.
16. Kasper DL. Harrison’s Principles of internal medicine. 6th. New York: McGraw-Hill Companies;2005. p. 2153–4.
17. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III): Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285:2486–97.
18. Guillen-Monterrubio OM, Hartikainen J, Taskinen K, Saari KM. Quantitative determination of aqueous flare and cells in healthy eyes. Acta Ophthalmol Scand. 1997; 75:58–62.
19. Klein R, Klein BE, Moss SE. . Four year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989; 107:244–9.
20. Cho YY, Kim CS, Ji NC. Relationship of diabetic retinopathy and glycosylated hemoglobin (HbA1c). J Korean Ophthalmol Soc. 1994; 35:552–7.
21. Sjolie AK. Blood pressure and retinopathy in insulin treated diabetic patients with early onset. Acta Ophthalmol. 1985; 173:S48–9.
22. Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood pressure. New Eng J Med. 1980; 302:645–50.

23. Jerneld B, Algvere P. Proteinuria and blood glucose levels in a population with diabetic retinopathy. Am J Ophthalmol. 1987; 104:283–9.

24. Weber B, Burger W, Harman R. . Risk factors for the development of retinopathy in children and adolescent with type I diabetes mellitus. Diabetologia. 1986; 29:23–9.
25. Klein BE, Moss SE, Klein R, Surawicz TS. Relationship of serum cholesterol to retinopathy and hard exudates: The Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 1991; 98:1261–5.
26. Chew Ey, Klein ML, Ferris FL. 3rd. . For the ETDRS research group. Association of elevated serum lipid levels with retinal hard exudates in diabetic retinopathy. Arch Ophthalmol. 1996; 114:1079–84.
Go to : 
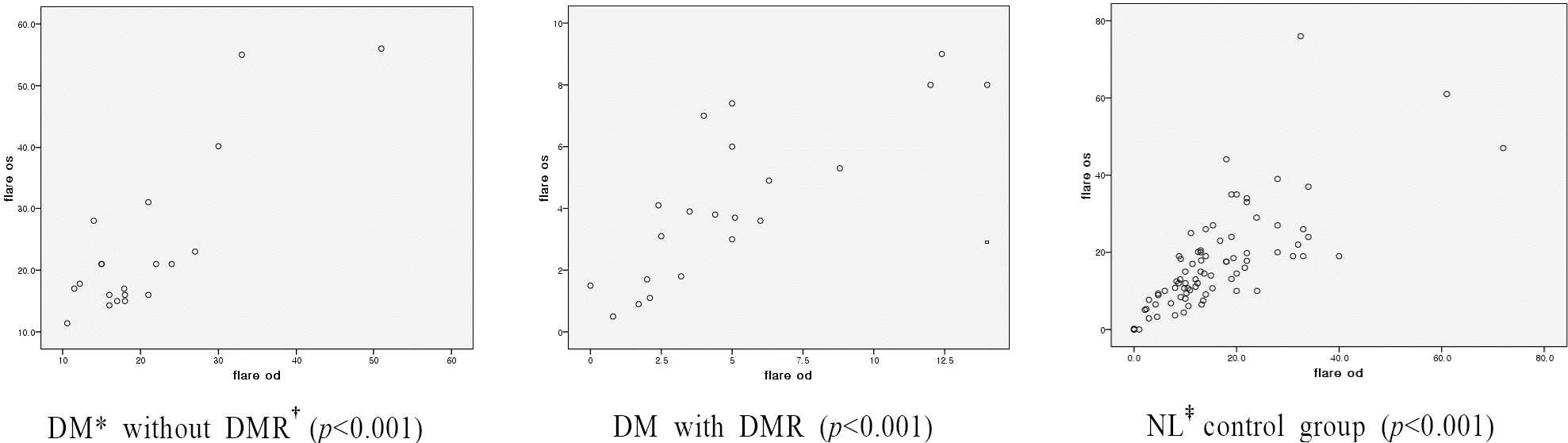 | Figure 1.Correlations of flare photometer values (ph/ms) between the right and left eyes in 3 groups. * DM=diabetes mellitus;† DMR=diabetic retinopathy; ‡ NL=normal. |
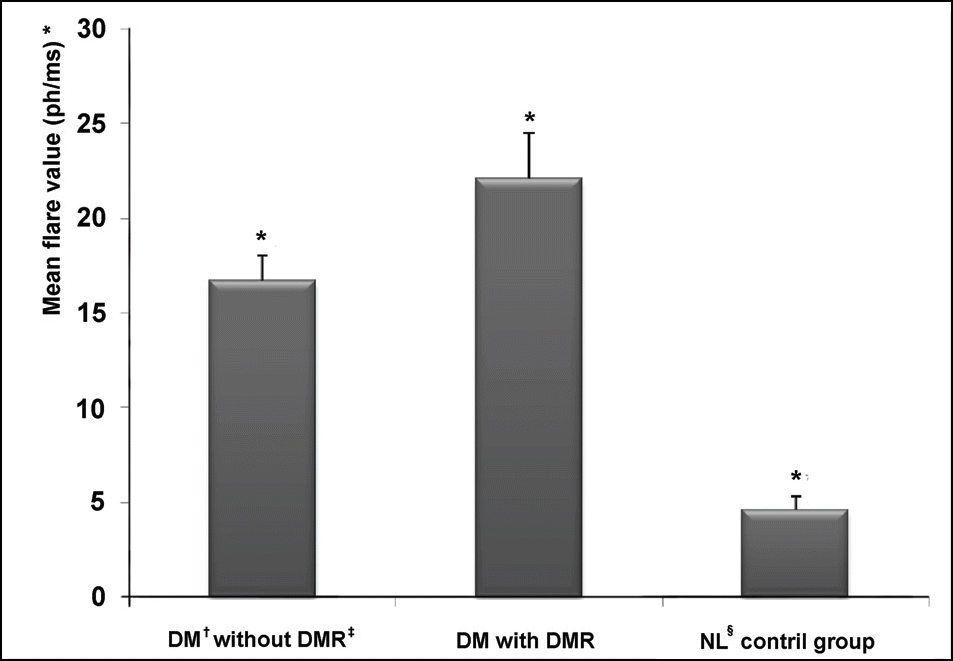 | Figure 2.Comparison of mean flare photometer values among 3 groups. (one-way ANOVA, p<0.001). † DM=diabetes mellitus; § NL=normal. * (Mean±standard ‡DMR=diabetic retinopathy; deviation) is (16.7±11.8 ph/ms) in DM without DMR, (22.1±10.6 ph/ms) in DM with DMR, and (4.6±3.1 ph/ms) in normal control group. |
Table 1.
Correlations of flare photometer values of diabetic patients without diabetic retinopathy with risk factors of diabetic retinopathy
| Risk factors of diabetic retinopathy | Correlations with flare photometer values (ph/ms) | |
|---|---|---|
| Pearson correlation coefficient | P value | |
| Age | 0.17 | 0.12 |
| Systolic BP∗ | -0.20 | 0.09 |
| Diastolic BP | -0.13 | 0.26 |
| DM† duration (years) | 0.05 | 0.65 |
| FPG‡(mg/dL) | -0.02 | 0.88 |
| 2-h PG§ (mg/dL) | -0.01 | 0.92 |
| HbA1c† (%) | -0.16 | 0.18 |
| Albuminuria (mg/L) | 0.01 | 0.95 |
| Total cholesterol (mg/dL) | 0.07 | 0.54 |
| Triglyceride (mg/dL) | -0.07 | 0.54 |
| HDL# cholesterol (mg/dL) | 0.23 (∗) | 0.05 |
| LDL∗∗ cholesterol (mg/dL) | -0.01 | 0.94 |
Table 2.
Comparison of mean flare photometer values between well- and poorly-controlled state
| Clinical parameters | Flare photometer value (ph/ms, Mean±SD∗) | P value | |
|---|---|---|---|
| well-controlled state | poorly-controlled state | ||
| HTN† | 17.23±10.98 | 16.00±14.12 | 0.68 |
| FPG‡(mg/L) | 18.00±14.40 | 16.70±09.96 | 0.66 |
| 2-h PG§ (mg/L) | 17.69±12.31 | 16.90±11.51 | 0.80 |
| HbA1c∏ (%) | 18.00±14.41 | 16.00±09.82 | 0.49 |
| Total cholesterol (mg/L) | 17.46±11.85 | 22.90±05.80 | 0.52 |
| Triglyceride (mg/L) | 18.00±12.50 | 15.64±09.40 | 0.50 |
| HDL# cholesterol (mg/L) | 17.92±12.29 | 16.83±11.53 | 0.72 |
| LDL∗∗ cholesterol (mg/L) | 17.82±11.91 | 13.48±18.35 | 0.62 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download