Abstract
Purpose
To investigate the long-term therapeutic effect and safety of repeated intravitreal bevacizumab injections for managing choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD).
Methods
Clinical data of 14 eyes of 14 patients who were treated with repeated (3 times or more) intravitreal bevacizumab injections for secondary to AMD and followed up for 12 months were collected. Patients were treated with 1.25 mg of intravitreal bevacizumab. Bevacizumab was injected at 6-week intervals. Reinjection was performed with the same method according to the physician’s decision.
Results
Mean visual acuity change increased by 4.0 lines from 1.13±0.47 to 0.73±0.44 (LogMAR). Mean central retinal thickness change decreased by 114.8 µm from 240.3±123.8 µm to 125.5±39.4 µm. Visual acuity improved statistically significantly at 12 weeks after first intravitreal bevacizumab injections and was maintained for 12 months. Central retinal thickness decreased statistically significantly at 6 weeks after the first intravitreal bevacizumab injection and was maintained for 12 months. During the 12 months of 4 intravitreal bevacizumab injections, mean visual acuity change increased by 4.0 lines at 12 months and 4.4 lines at 7 months.
Go to : 
References
1. Ferris FL. 3rd. Senile macular degeneration: Review of epidemiologic features. Am J Epidemiol. 1983; 118:132–51.

2. Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102:1640–2.
3. Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988; 32:375–413.

4. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1132–42.
5. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001; 119:198–207.
6. Verteporfin In Photodynamic Therapy Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001; 131:541–60.
7. Lee HJ, Kang JE, Lee JH. A Case report of acute visual decrease due to choroidal nonperfusion after photodynamic therapy. J Korean Ophthalmol Soc. 2005; 46:1411–8.
8. Leung DW, Cachianes G, Kuang WJ. . Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246:1306–9.

9. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992; 359:845–8.

10. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.

11. Spaide RF, Laud K, Fine HF. . Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.

12. Yoganathan P, Deramo VA, Lai JC. . Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006; 26:994–8.

13. Goff MJ, Johnson RN, McDonald HR. . Intravitreal bevacizumab for previously treated choroidal neovascularization from age-related macular degeneration. Retina. 2007; 27:432–8.

14. Ladas ID, Kotsolis AI, Papakostas TD. . Intravitreal bevacizumab combined with photodynamic therapy for the treatment of occult choroidal neovascularization associated with serous pigment epithelium detachment in age-related macular degeneration. Retina. 2007; 27:891–6.

15. Stifter E, Michels S, Prager F. . Intravitreal Bevacizumab Therapy for Neovascular Age-related Macular Degeneration with Large Submacular Hemorrhage. Am J Ophthalmol. 2007; 144:886–92.

16. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

17. Avery RL, Pieramici DJ, Rabena MD. . Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

18. Chen CY, Wong TY, Heriot WJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration: a short-term study. Am J Ophthalmol. 2007; 143:510–2.

19. Lazic R, Gabric N. Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007; 245:68–73.

20. Falkenstein IA, Cheng L, Morrison VL. . Standardized visual acuity results associated with primary versus secondary bevacizumab (avastin) treatment for choroidal neovascularization in age-related macular degeneration. Retina. 2007; 27:701–6.

21. Rosenfeld PJ, Brown DM, Heier JS. . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

22. Brown DM, Kaiser PK, Michels M. . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

23. Bakri SJ, Snyder MR, Reid JM. . Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.

24. Bakri SJ, Snyder MR, Reid JM. . Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114:2179–82.

Go to : 
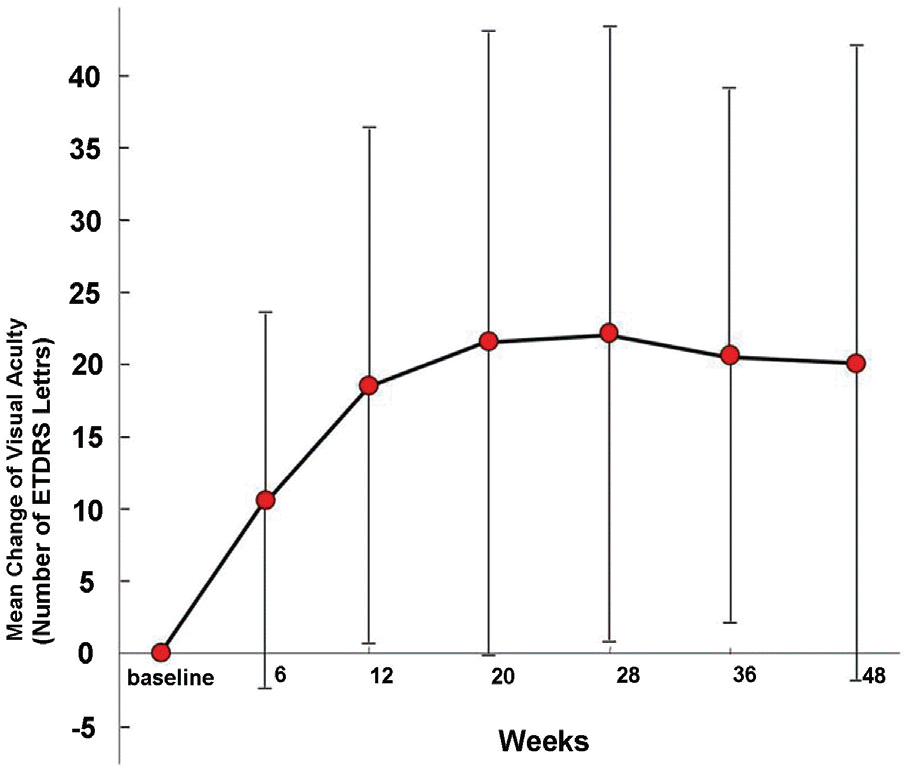 | Figure 1. Mean (±SD) change in visual acuity letter scores through 48 weeks (5 letters=1 line). |
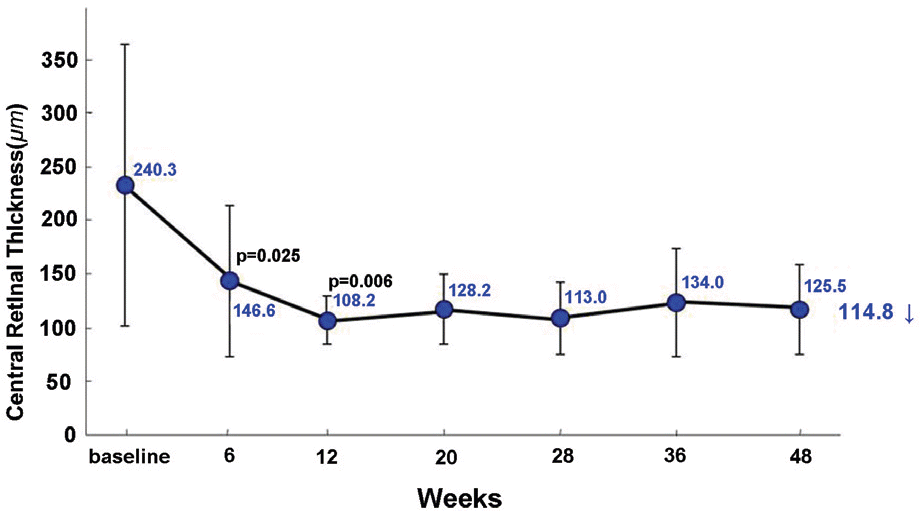 | Figure 2. Change in central retinal thickness from baseline to 48 weeks in all treated eyes. |
Table 1.
Patient demographics, changes in visual acuity and number of injections
| Patients | Sex | Age | Pretreatment VA∗ (LogMAR) | VA∗ at 12 month (LogMAR) | VA∗ change ( LogMAR) | Number of injections | Lesion type | GLD† |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 79 | 0.76 | 0.30 | -0.46 | 7 | Occult | 3120 |
| 2 | F | 77 | 1.40 | 0.82 | -0.58 | 6 | Occult | 3850 |
| 3 | M | 61 | 0.86 | 0.80 | -0.06 | 3 | MC‡ | 3500 |
| 4 | F | 72 | 0.86 | 0.70 | -0.16 | 3 | Occult | 3210 |
| 5 | M | 77 | 1.10 | 1.10 | 0 | 3 | MC‡ | 3450 |
| 6 | M | 86 | 0.74 | 0.10 | -0.64 | 3 | PC§ | 1820 |
| 7 | M | 58 | 0.64 | 0.30 | -0.34 | 3 | Occult | 2410 |
| 8 | M | 69 | 2.00 | 0.40 | -1.6 | 5 | Occult | 1010 |
| 9 | M | 59 | 1.80 | 1.00 | -0.8 | 5 | Occult | 1250 |
| 10 | F | 69 | 0.52 | 0.52 | 0 | 3 | PC§ | 2010 |
| 11 | F | 65 | 1.26 | 1.26 | 0 | 3 | Occult | 2750 |
| 12 | M | 80 | 1.20 | 1.04 | -0.16 | 5 | Occult | 4650 |
| 13 | M | 69 | 0.90 | 0.22 | -0.68 | 4 | Occult | 2520 |
| 14 | F | 66 | 1.80 | 1.70 | -0.1 | 3 | Occult | 1170 |
| Mean | 70.5 | 1.13 | 0.73 | -0.40 | 4.0 | 2623 |
Table 2.
Patient demographics, changes in central retinal thickness and number of injections
| Patients | Sex | Age | Pretreatment CRT∗ (µm) | CRT∗ at 12 month (µm) | CRT∗ change (µm) | Number of injections | Lesion type | GLD‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 79 | 273 | 174 | -99 | 7 | Occult | 3120 |
| 2 | F | 77 | 179 | 180 | 1 | 6 | Occult | 3850 |
| 3 | M | 61 | 343 | 101 | -242 | 3 | MC‡ | 3500 |
| 4 | F | 72 | 218 | 74 | -144 | 3 | Occult | 3210 |
| 5 | M | 77 | 191 | 113 | -78 | 3 | MC‡ | 3450 |
| 6 | M | 86 | 332 | 140 | -192 | 3 | PC§ | 1820 |
| 7 | M | 58 | 547 | 121 | -426 | 3 | Occult | 2410 |
| 8 | M | 69 | 333 | 212 | -121 | 5 | Occult | 1010 |
| 9 | M | 59 | 115 | 126 | 11 | 5 | Occult | 1250 |
| 10 | F | 69 | 241 | 107 | -134 | 3 | PC§ | 2010 |
| 11 | F | 65 | 78 | 85 | 7 | 3 | Occult | 2750 |
| 12 | M | 80 | 148 | 132 | -16 | 5 | Occult | 4650 |
| 13 | M | 69 | 263 | 99 | -164 | 4 | Occult | 2520 |
| 14 | F | 66 | 103 | 93 | -10 | 3 | Occult | 1170 |
| Mean | 70.5 | 240.3 | 125.5 | -114.8 | 4.0 | 2623 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download