Abstract
Purpose
To clinically establish the effectiveness and safety of bevacizumab on recurrent pterygium. Methods: Twenty patients with recurrent pterygium were given a subconjunctival injection of 0.3 cc bevacizumab, and were evaluated for periodic clinical results at 1 week, 2 weeks, 4 weeks, and every month thereafter. The patients were also evaluated for clinical results and complications.
Results
Of recurrent pterygium patients with bevacizumab injection, the conjunctival injection decreased maximally after 1 to 2 weeks, but significantly increased at 4 weeks (above the lowest level measured at 1 to 2 weeks), and no patient presented conjunctival injection above the pre-injection level at 3 months, except in 2 cases. Two weeks after the injection, ICG anterior segment angiography revealed a significant decrease (30.14+17.69%) in vessel thickness of the pterygium 2 weeks after the bevacizumab injection compared to before the injection. There had been no cases of progression of pterygium, and no ocular or systemic complications due to bevacizumab.
Conclusions
As shown above in the results, subconjunctival injection of 0.3 cc bevacizumab decreased the conjunctival injection and effectively suppressed any further progression of pterygium. Thus, bevacizumab subconjunctival injection appears to be effective in recurrent pterygium treatment instead of surgical methods.
Go to : 
References
2. Wong W. A hypothesis on the pathogenesis of pterygium. Ann Ophthalmol. 1978; 10:303–8.
3. Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993; 77:734–9.

4. Kria L, Ohira A, Amemiya T. Immunohisto chemical localization of basic fibroblast growth factor, platelet derived grwth factor, transforming growth factor-ß and tumor necrosis factor-α in the pterygium. Acta Histochem. 1996; 98:195–201.
5. Kria L, Ohira A, Amemiya T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998; 236:702–8.

6. Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001; 42:1963–8.
7. Lee DH, Cho HJ, Kim JT. . Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001; 20:738–42.

8. Marcovich AL, Morad Y, Sandbank J. . Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002; 25:17–22.

9. Aspiotis M, Tsanou E, Gorezis S. . Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye. 2007; 21:1095–101.

10. Gebhardt M, Mentlein R, Schaudig U. . Differential expression of vascular endothelial growth factor implies the limbal origin of pterygia. Ophthalmology. 2005; 112:1023–30.

11. Jin J, Guan M, Sima J. . Decreased pigment epithelium- derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003; 22:473–7.
12. Ferrara N, Hillan KJ, Novotny W. Bevacizumab (avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005; 333:328–35.

13. Lazic R, Gabric N. Intravitreally administered bevacizumab (avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007; 245:68–73.
14. Jorge R, Costa RA, Calucci D. . Intravitreal bevacizumab (avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina. 2006; 26:1006–13.

15. Iliev ME, Domig D, Wolf-Schnurrbursch U. . Intravitreal bevacizumab (avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006; 142:1054–6.

16. Manzano RP, Peyman GA, Khan P. . Inhibition of experimental corneal neovascularisation by bevacizumab (avastin). Br J Ophthalmol. 2007; 91:804–7.

17. DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascualrization. Arch Ophthalmol. 2007; 125:834–6.
18. Bock F, Onderka J, Dietrich T. . Bevacizumab as a potent inhibitior of inflammatory corneal angiogenesis and lymphangio- genesis. Invest Ophthalmol Vis Sci. 2007; 48:2545–52.
19. Hosseini H, Nejabat M. A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses. 2007; 68:799–801.

20. Sunderkotter C, Roth J, Sorg C. Immunohistochemical detection of β-FGF and TNF-α in the course of inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1990; 137:511–5.
21. Beck L Jr, D’Amore PA. Vascular development. cellular and molecular regulation. FASEB J. 1997; 11:365–73.

22. Enholm B, Paavonen K, Ristimaki A. . Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997; 14:2475–83.

23. Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995; 270:19761–6.

24. Waltenberger J, Mayr U, Pentz S, Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation. 1996; 94:1647–54.

25. Bakri SJ, Snyder MR, Reid JM. . Pharmacokinetics of intravitreal bevacizumab (avastin). Ophthalmology. 2007; 114:855–9.

26. Weijtens O, Feron EJ, Schoemaker RC. . High concentration of dexamethasone in aqueous and vitreous after subconjuntival injection. Am J Ophthalmol. 1999; 128:192–7.
Go to : 
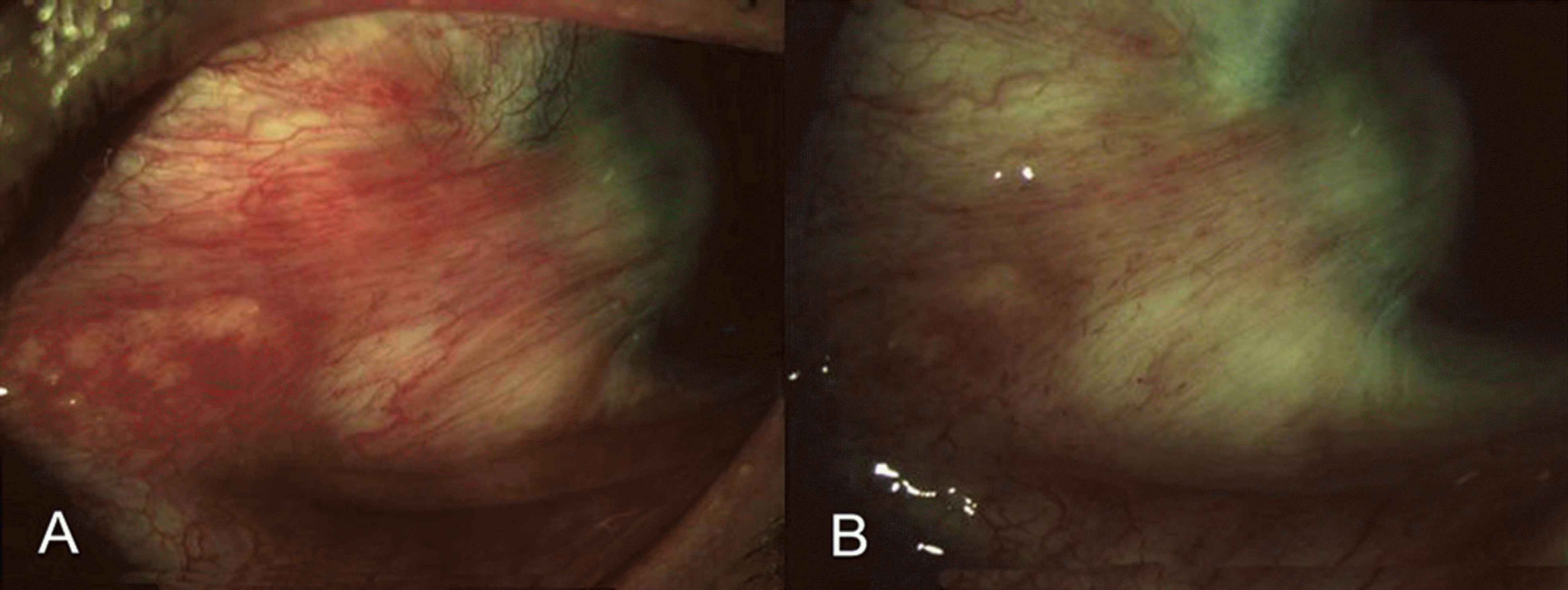 | Figure 1. Clinical appearance of the conjunctival injection before (A) and after (B) 0.3 cc subconjunctival injection of 0.3 cc of bevacizumab. (A) Before treatment: Severe conjunctival hyperemia of recurrent pterygium. (B) One week after treatment: Conjunctival injection and vascularization of recurrent pterygium markedly decreased. |
 | Figure 2. Changes in degree of conjunctival injection in 3 cases of recurrent pterygium before subconjunctival injection of bevacizumab and thereafter: (1, pre-injection; 2, 2 weeks; 3, 2 months; 4, 5 months). (ABCD-1) Severe conjunctival injection due to recurrent pterygium before subconjunctival injection of bevacizumab. (ABCD-2) Two weeks after subconjunctival injection of bevacizumab presents maximal reduction in both conjunctival injection and conjunctival vessel diameter. (ABCD-3) Two months after subconjunctival injection of bevacizumab, relative increase in conjunctival injection and conjunctival vessel diameter compared to ABCD-2 (2 weeks). (ABCD-4) Five months after subconjunctival injection of bevacizumab, stabilized conjunctival injection, without further progression of pterygium. |
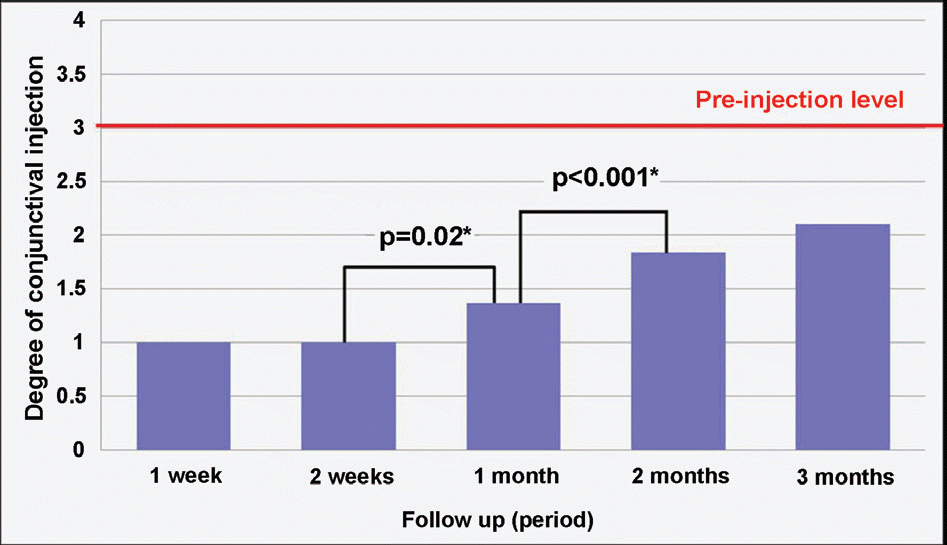 | Figure 3. Progressive changes in the level of conjunctival injection after bevacizumab injection in recurrent pterygium. * The chart indicates significant increase in conjunctival injection 1 month after subconjunctival bevacizumab injection ( p<0.05, Wilcoxon signed ranks test). However, there were no cases of increased conjunctival injection up to 3 months follow-up, compared to pre- injection level except in 2 eyes. The scoring system of conjunctival injection: Lever 1; When conjunctival injection has reached the rock-bottom after bevacizumab injection. Level 2; Level between 1~3. Level 3; Similar level of conjunctival injection to the degree before bevacizumab injection. Level 4; Shows increase of conjunctival injection compared to the degree before bevacizumab injection. |
 | Figure 4. Rare cases (2 cases) of rebound conjunctival injection following subconjunctival bevacizumab injection for recurrent pterygium, and alleviation of conjunctival injection following reinjection. (AB-1) Recurrent pterygium before subconjunctival injection of bevacizumab. (AB-2) Minimized conjunctival injection 2 weeks after subconjunctival injection of bevacizumab. (AB-3) Markedly increased conjunctival injection (compared to pre-injection) presented at 2 months after the initial injection. (AB-4) Stabilization of conjunctival injection 3 months after reinjection of subconjunctival bevacizumab.SS |
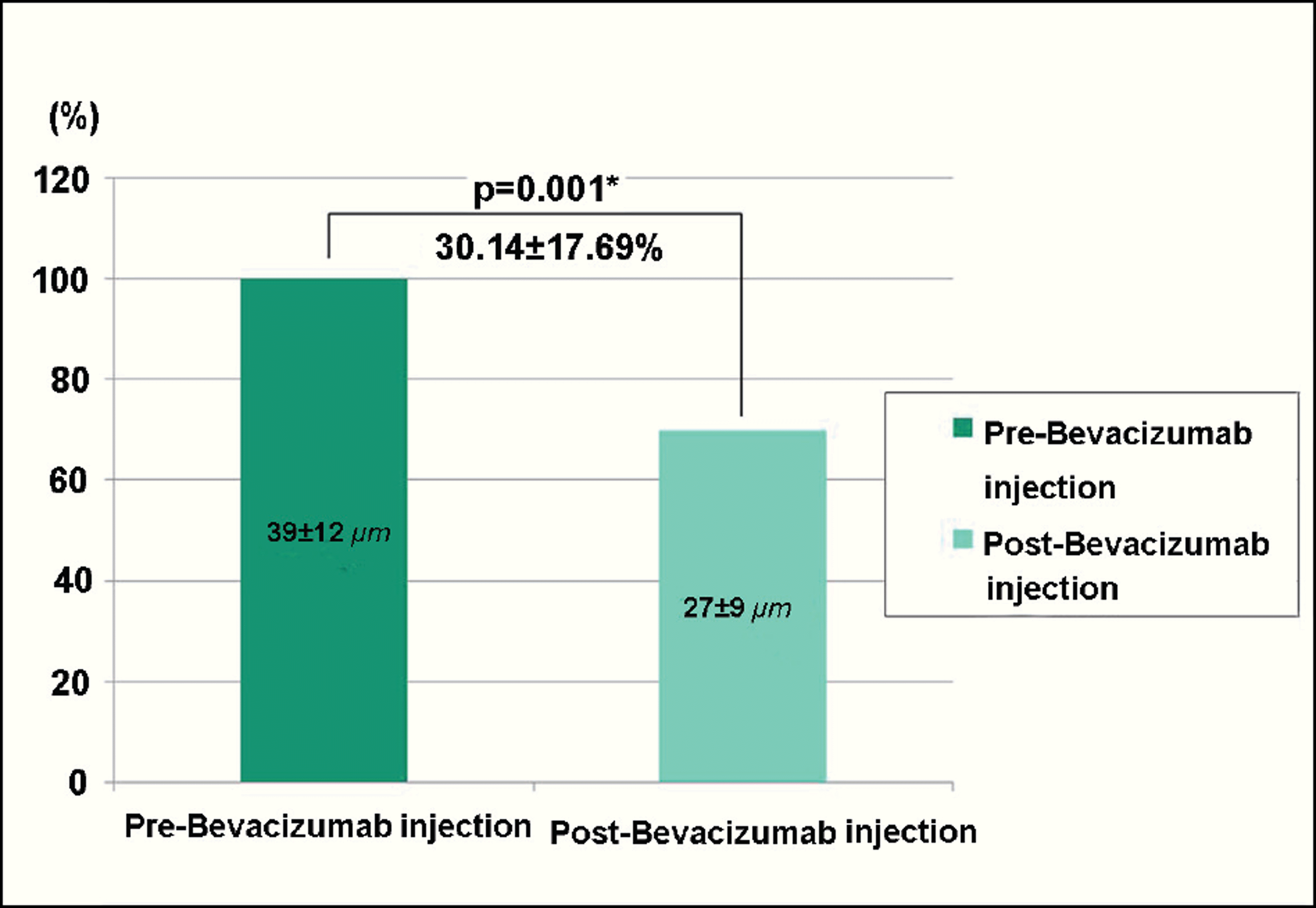 | Figure 5. The change in vessel thickness of the pterygium before and after subconjunctival bevacizumab injection in recurrent pterygium. * The vessel thickness of the pterygium decreased significantly in comparison between before (39±12µm) and after (27±9 µm) the bevacizumab injection (30.14±17.69%, p<0.05, paired t-test). |
 | Figure 6. Anterior segment photographs (A-1, A-2) and anterior segment ICG angiography (B-1, B-2) before and after subconjunctival bevacizumab injection. (B-1 and B-2) shows a decrease in vessel thickness of the pterygium after subconjunctival injection of bevacizumab (red arrow). The small vessel thickness of the pterygium shows regression after the subconjunctival injection of bevacizumab (yellow circle). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download