Abstract
Purpose
To evaluate the results of transpupillary thermotherapy combined with chemotherapy for the treatment of retinoblastoma.
Methods
Retinoblastoma patients treated with chemotherapy and transpupillary thermotherapy from November, 2004 to October, 2007 were retrospectively reviewed. Local tumor control was assessed in terms of regression or recurrence, at 1 month after chemotherapy and each session of thermotherapy, as well as the final follow-up period.
Result
Fifty-nine tumors were treated in 15 eyes of 11 children. Age at diagnosis was 7.4±6.9 months. Mean tumor diameter at the time of diagnosis was 2.2±2.1 disc diameters (DD) and mean tumor diameter at the time of initial thermotherapy was 1.8±1.7DD. Mean number of thermotherapy sessions for each tumor was 1.3±0.5. Total tumor regression was obtained for 96.6% of tumors after a mean follow-up of 22.3±10.7 months. The initial tumor size before thermotherapy was significantly smaller in the group of tumors showing total regression after the first session of thermotherapy. The time required for total regression after initial thermotherapy was related to the initial tumor size before thermotherapy.
Go to : 
References
1. Abramson DH, Niksarli K, Ellsworth RM, Servodidio CA. Changing trends in the management of retinoblastoma: 1951-1965 vs 1966-1980. J Pediatr Ophthalmol Strabismus. 1994; 31:32–7.

2. Shields CL, Shields JA, De Potter P. New treatment modalities for retinoblastoma. Curr Opin Ophthalmol. 1996; 7:20–6.

3. Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998; 105:573–9.
4. Yu YS, Kim IH, Ahn HS. J Korean Ophthalmol Soc. 1996; 37:1349–53.
5. Fontanesi J, Pratt CB, Hustu HO. Use of irradiation for therapy of retinoblastoma in children more than 1 year old: the St. Jude Children’s Research Hospital experience and review of literature. Med Pediatr Oncol. 1995; 24:321–6.
6. Shields CL, Shields JA, Needle M. . Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology. 1997; 104:2101–11.

7. Gallie BL, Budning A, DeBoer G. . Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996; 114:1321–8.

8. Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996; 114:1339–43.

9. Shields CL, Honavar SG, Meadows AT. . Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002; 133:657–64.
10. Journée-de Korver JG, osterhuis JA, Kakebeeke-Kemme HM, de Wolff-Rouendaal D. Transpupillary thermotherapy by infrared irradiation of choroidal melanoma. Doc Ophthalmol. 1992; 82:185–91.
11. Oosterrhuis JA, Journee-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995; 113:315–21.

12. Tsai TH, Yang CM, Yang CH. . Transpupillary thermotherapy for the treatment of choroidal neovascularization in age-related macular degeneration in Taiwan. Eye. 2007; 21:721–6.

13. Lanzetta P, Michieletto P, Pirracchio A, Bandello F. Early vascular changes induced by transpupillary thermotherapy of choroidal neovascularization. Ophthalmology. 2002; 109:1098–104.

14. Rishi P, Sharma T. Transpupillary thermotherapy for large-sized subfoveal circumscribed choroidal hemangioma. Retina. 2006; 26:974–6.

15. Gündüz K. Transpupillary thermotherapy in the management of circumscribed choroidal hemangioma. Surv Ophthalmol. 2004; 49:316–27.

16. Subramanian ML, Reichel E. Current indications of transpupillary thermotherapy for the treatment of posterior segment diseases. Curr Opin Ophthalmol. 2003; 14:155–8.

17. Abramson DH, Schefler AC. Transpupillary thermotherapy as initial treatment for small intraocular retinoblastoma: technique and predictors of success. Ophthalmology. 2004; 111:984–91.
18. Ozdek S, Urgancioglu B, Turkcu UO, Bilgihan A. A possible mechanism for transpupillary thermotherapy: nitric-oxide-related cellular damage. Can J Ophthalmol. 2007; 42:609–12.

19. Lagendijk JJ. A microwave heating technique for the hyperthermic treatment of tumours in the eye, especially retinoblastoma. Phys Med Biol. 1982; 27:1313–24.

20. Lumbroso L, Doz F, Urbieta M. . Chemothermotherapy in the management of retinoblastoma. Ophthalmology. 2002; 109:1130–6.
21. Murphree AL, Villablanca JG, Deegan WF. . Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996; 114:1348–56.

22. Lin HY, Lee FL, Ko YC. Hsieh YL. Multifocal retinoblastomas treated with transpupillary thermotherapy. Zhonghua Yi Xue Za Zhi (Taipei). 2002; 65:41–4.
23. Oh JW, Yun IH. Transpupillary thermotherapy in circumscribed choroidal hemangioma. J Korean Ophthalmol Soc. 2003; 44:992–7.
24. Kim US, Yu SY, Kim SW, Kwak HW. Five cases of transpupillary thermotherapy for intraocular tumors. J Korean Ophthalmol Soc. 2003; 44:2942–9.
25. Shields CL, Santos MC, Diniz W. . Thermotherapy for retinoblastoma. Arch Ophthalmol. 1999; 117:885–93.

26. Antoneli CB, Ribeiro KC, Steinhorst F. . Treatment of retinoblastoma patients with chemoreduction plus local therapy: experience of the AC Camargo Hospital, Brazil. J Pediatr Hematol Oncol. 2006; 28:342–5.

27. Brichard B, De Bruycker JJ, De Potter P. . Combined chemotherapy and local treatment in the management of intraocular retinoblastoma. Med Pediatr Oncol. 2002; 38:411–5.

28. Lumbroso L, Doz F, Levy C. . Diode laser thermotherapy and chemothermotherapy in the treatment of retinoblastoma. J Fr Ophtalmol. 2003; 26:154–9.
29. Mashayekhi A, Cater J. Macular retinoblastoma managed with chemoreduction: analysis of tumor control with or without adjuvant thermotherapy in 68 tumors. Arch Ophthalmol. 2005; 123:765–73.
30. Rodriguez-Galindo C, Chantada GL, Haik BG, Wilson MW.Treatment of retinoblastoma: current status and future perspectives. Curr Treat Options Neurol. 2007; 9:294–307.

31. Goldman S, Strauss LC. Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology. 1998; 105:1579–81.

Go to : 
 | Figure 1.Multiple retinoblastomas treated with initial chemotherapy and thermotherapy for all the tumors. (A) and (B) Retinoblastomas before any treatment. Initial base diameters were 1.5DD (A, white arrow) and 4DD (B, hollow arrow). Combined chemotherapy and thermotherapy were applied to both masses. (C) One month after initial thermotherapy combined with systemic chemotherapy, both masses showed total regression; DD=disc diameter. |
 | Figure 2.Multiple retinoblastomas treated with initial chemotherapy and further local thermotherapy for one tumor. (A) Two months after initial chemotherapy, showing no regression of the inferonasal 3DD mass, and partial regression of the 6DD macular mass. Thermotherapy was applied on the inferonasal mass (white arrow). (B) Immediately after thermotherapy, capillary dilatation and hemorrhage was found of the overlying vessels of the inferonasal mass (white arrow). (C) One month after thermotherapy, the inferonasal mass was much flattened down and showed nearly total regression; DD=disc diameter. |
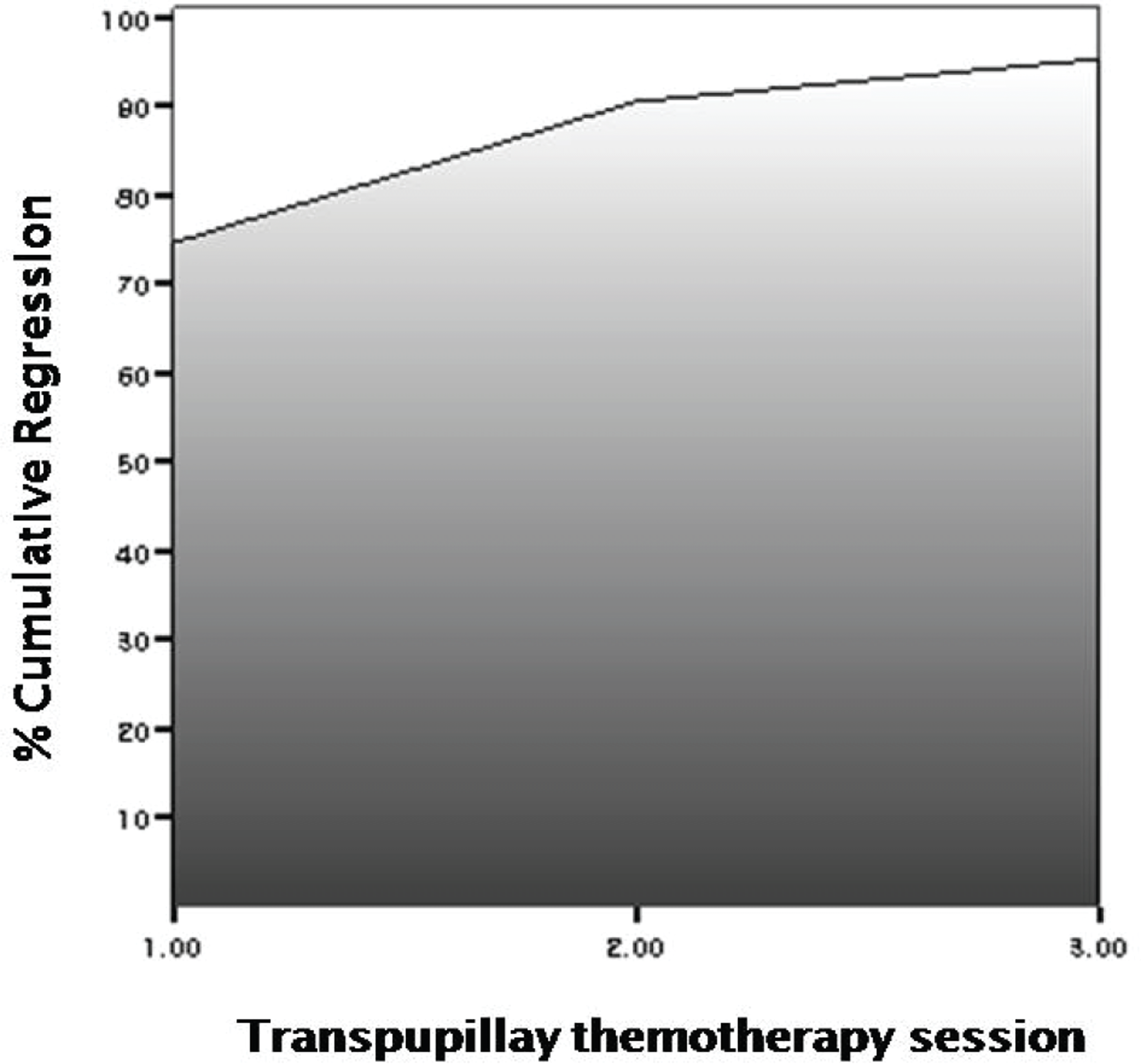 | Figure 3.Cumulative regression rate after increased number of transpupillary thermotherapy sessions combined with chemoreduction. A cumulative total of 72.9% (43/59), 89.8%(53/39), 96.6% (57/59) of tumors showed total regression after 1, 2, and 3 sessions of thermotherapy combined with chemotherapy. |
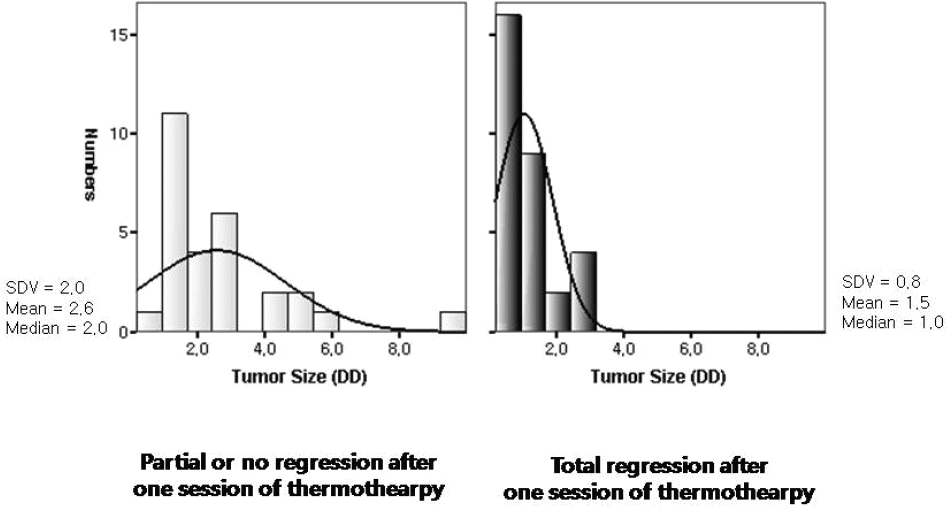 | Figure 4.The distribution of initial tumor size (base diameter) before thermotherapy, according to the response pattern after one session of thermotherapy combined with systemic chemotherapy. Initial tumor size before thermo- therapy was smaller in the group of tumors that showed total regression after one session of thermotherapy combined with chemotherapy ( p=0.01); DD=disc diameter; SDV=standard deviation. |
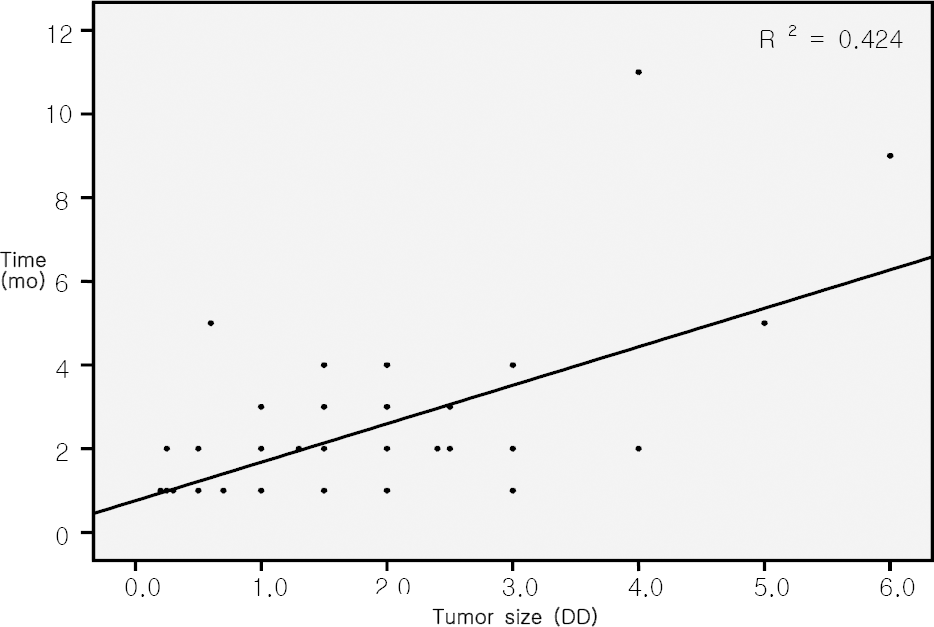 | Figure 5.Scattergram showing the relationship of initial tumor size (base diameter) before thermotherapy and the time to total tumor regression after initial thermotherapy (mo). The best fit line portrays the positive correlation with statistical significance (R2=0.424, p<0.01); DD=disc diameter; mo=months. |
Table 1.
Patient characteristics
| Total number of patients | 11 | |
| Eyes | 15 | |
| Gender | Male | 7 (63.6%) |
| Female | 4 (36.4%) | |
| Bilaterality of retinoblastoma | Bilateral | 10 (90.9%) |
| Unilateral | 1 (9.1%) | |
| Age at diagnosis (mo) | 7.4±6.9 (1-21) | |
| Follow-up (mo) after diagnosis | 22.3±10.7 (7-38) | |
| Bilaterality of thermotherapy | Bilateral | 4 (36.4%) |
| Unilateral* | 7 (63.6%) | |
| Status of eye | Enucleated | 1 (6.7%) |
| Preserved with active disease | 0 (0%) | |
| Preserved with no active disease | 14 (93.3%) | |
| Average tumor numbers treated with thermotherapy per eye† | 4.1±3.0 (1-12) | |
Table 2.
Tumor characteristics
| Total number of tumors | 59 | |
| Tumor size (DD) | Initial size at diagnosis, before chemotherapy | 2.2±2.1 (0.2-10) |
| Initial size before thermotherapy | 1.8±1.7 (0.2-10) | |
| Thermotherapy sessions | 1.3±0.5 (1-3) | |
| Initial thermotherapy after 1st cycle of chemotherapy* (mo) | 3.3±4.2 (0-13) | |
| Time to total regression after last thermotherapy†(mo) | 1.5±0.9 (1-5) | |
| Follow-up after total regression of tumor†(mo) | 12.5±9.4 (0-32) | |
| Tumor R-E classification | I-II | 22 (37.3%) |
| III-IV | 37 (62.7%) | |
| Tumor laterality | Right eye | 39 (66.1%) |
| Left eye | 20 (33.9%) | |
| Tumor location | Superior | 32 (54.2%) |
| Inferior | 27 (45.8%) | |
| Temporal | 22 (37.3%) | |
| Nasal | 37 (62.7%) | |
| Posterior to equator | 48 (81.4%) | |
| Anterior to equator | 11 (18.6%) | |
Table 3.
Power settings and durations of transpupillary thermotherapy (TTT) for increasing number of with systemic chemotherapy (CTx).TTT sessions combined
| TTT | 1 st session | 2 nd session | 3 rd session | Total |
|---|---|---|---|---|
| Total tumors | 59 | 14 | 2 | 59 |
| Tumor size (DD) before CTx* | 2.2±2.1 (0.2-10) | 3.5±2.4 (1.5-10) | 6.0±1.4 (5-7) | 2.2±2.1 (0.2-10) |
| Tumor size (DD) before TTT† | 1.8±1.7 (0.2-10) | 3.1±2.5 (1-10) | 5.5±0.7 (5-6) | 1.8±1.7 (0.2-10) |
| Power (mW) | 236±87 (80-600) | 209±37 (175-300) | 150±71 (100-200) | 229±82 (80-600) |
| Duration (sec) | 89±131 (4-736) | 47±33 (4-105) | 33±4 (30-36) | 84±120 (4-736) |
| Energy (J) | 28±68 (1-464) | 9±7 (1-23) | 8±6 (4-12) | 25±62 (1-464) |
Table 4.
Predictive variables of insufficient response after one session of thermotherapy combined with chemoreduction
| Variable | p-value |
|---|---|
| Gender (Male/Female*) | 0.60 |
| Age at diagnosis | 0.39 |
| Bilaterality (Bilateral/Unilateral*) | 0.48 |
| R-E classification (III-IV/ I-II*) | 0.17 |
| Tumor location (Right/Left*) | 0.79 |
| Tumor location (Superior/Inferior*) | 0.01† |
| Tumor location (Temporal/Nasal*) | <0.01† |
| Tumor location (Posterior/Anterior*) | 0.88 |
| Initial tumor Size | <0.01‡ |
| Initial tumor numbers per eye | 0.60 |
| Initial tumor size per eye | 0.06 |
| Energy applied per 1DD3 | 0.09 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download