Abstract
Purpose
This study evaluated the effect and safety of photodynamic therapy (PDT) with verteporfin retreatment on patients with corneal neovascularization.
Methods
Corneal neovascularization was induced with a silk suture of the corneal stroma in 24 white rabbits (48 eyes). Four rabbits were examined histologically before performing PDT. Ten rabbits were chosen randomly, one eye from each rabbit was treated with PDT at 50 J/cm2, and the other eye was used as a control. Both eyes of the remaining 10 rabbits were treated with PDT at 150 J/cm2. One week later, one eye was further retreated with PDT at the same intensity. The area of corneal neovascularization was measured and evaluated histologically using light and electron microscopies.
Results
The least neovascularized area was observed in the retreated group ( P=0.04). The histologic examination revealed fewer new corneal vessels in the retreated group, but the corneal epithelium, stroma, and endothelium showed a normal appearance. Results from electron microscopic examination demonstrated thrombi as well as destruction and nuclear fragmentation of the vascular endothelial cells. However, there were no other changes in the cornea except for vascular endothelial cells, even in the retreated group.
Go to : 
References
1. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001; 12:242–9.

2. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998; 43:245–69.
3. Peyman GA, Kazi AA, Riazi-Esfahani M. . The effect of combinations of flubiprofen, low molecular weight heparin, and doxycycline on the inhibition of corneal neovascularization. Cornea. 2006; 25:582–5.
4. Thompson RW Jr, Price Mo, Bowers PJ, Price FW Jr. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003; 110:1396–1402.

5. Ambati BK, Joussen AM, Ambati J. . Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002; 120:1063–8.

6. Shao C, Sima J, Zhang SX. . Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest Ophthalmol Vis Sci. 2004; 45:1758–62.

7. Phillips K, Arffa R, Cintron C. . Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration, and neovascularization. Arch Ophthalmol. 1983; 101:640–3.

8. Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1999; 237:920–7.

9. Murata M, Shimizu S, Houriuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006; 244:205–9.

10. Mendelsohn AD, Lo GG, Stock EL, Schneck GL. Laser photocoagulation of feeder vessels in lipid keratopathy. Ophthalmic Surg. 1986; 17:502–8.

11. Gomer CJ. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. 1991; 54:1093–1107.

12. Primbs GB, Casey R, Wamser K. . Photodynamic therapy for corneal neovascualrization. Ophthalmic Surg Lasers. 1998; 29:832–8.
13. Bressler NM, Bressler SB, Fine SL. Neovascular (Exudative) age-related macular degeneration. Ryan SJ, Retina , editors. 4th. St Louis, London, Philadelphia, Toronto, Sydney: Mosby, Inc.;2005. v. 2. chap. 61.

15. Miller JW, Schmidt-Erfurth U, Sickenberg M. . Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117:1161–73.
16. Harding S. Photodynamic therapy in the treatment subfoveal choroidal neovascularization. Eye. 2001; 15:407–12.
17. Verteporfin in photodynamic therapy study group Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin 1-year results of a randomized clinical trial-VIP report no.1. Ophthalmology. 2001; 108:841–52.
18. Blumenkranz MS, Bressler NM, Bressler SB. . Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: three-year results of an open-label extension of 2 randomized clinical trials-TAP Report no. 5. Arch Ophthalmol. 2002; 120:1307–14.
19. Holzer MP, Solomon KD, Vroman DT. . Photodynamic therapy with verteporfin in a rabbit model of corneal neovacularization. Invest Ophthalmol Vis Sci. 2003; 44:2954–8.
20. Nah HJ, Yoon KC, Im WB. . Animal study of photodynamic therapy with verteporfin in corneal neovascualrization. J Korean Ophthalmol Soc. 2005; 46:707–15.
21. Yoon KC, Im SK, Oh HJ, Park YK. Two cases of photodynamic therapy with verteporfin in patients with corneal neovascularization. J Korean Ophthalmol Soc. 2006; 47:13–8.
22. Brooks BJ, Ambati BK, Marcus DM, Ratanasit A. Photodynamic therapy for corneal neovascularization and lipid degeneration. Br J Ophthalmol. 2004; 88:840.
23. Fossarello M, Peiretti E, Zucca I, Serra A. Photodynamic therapy of corneal neovascularization with verteporfin. Cornea. 2003; 22:485–8.

25. Wu PC, Liu CC, Chen CH. . Inhibition of experimental angiogenesis of cornea by somatostatin. Graefes Arch Clin Exp Ophthalmol. 2003; 241:63–9.

26. Selvasekar CR, Birbeck N, McMillan T. Review article: Photodynamic therapy and the alimentary tract. Aliment Pharmacol Ther. 2001; 15:899–915.
27. Pallikaris IG, Tslimbaris MK, IIiaki OE. . Effectiveness of corneal neovascularization photothrombosis using phthalocyanine and a diode laser (675 nm). Lasers Surg Med. 1993; 13:197–203.

28. Schmidt-Erfurth U, Hasan T, Schomacker K. . In vivo uptake of liposomal benzoporphyrin derivative and photothrombosis in experimental corneal neovascularization. Lasers Surg Med. 1995; 17:178–88.

29. Joussen AM, Kruse FE, Sinn H, Voelcker HE. Photothrombosis of corneal neovascularization with photosensitizers coupled to macromolecules. Lasers and Light. 1998; 8:211–9.
30. Porrini G, Giovannini A, Amato G, Ioni A. Photodynamic therapy of circumscribed choroidal haemangioma. Ophthalmology. 2003; 110:674–80.
31. Singh AD, Kaiser PK, Sears JE. . Photodynamic therapy of circumscribed choroidal haemangioma. Br J Ophthalmol. 2004; 88:1414–8.

32. Barbazetto IA, Lee TC, Rollins IS. . Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003; 135:898–9.

33. Parodi MB, Iacono P. Photodynamic therapy with verteporfin for anterior segment neovascularization in neovascular glaucoma. Am J Ophthalmol. 2004; 138:157–8.
34. Fossarello M, Peiretti E, Zucca I. . Photodynamic therapy of pterygium with verteporfin. Cornea. 2004; 23:330–8.

35. Elner SG, Elner VM, Pavilack MA. . Human and monkey corneal endothelium expression of low-density lipoprotein receptors. Am J Ophthalmol. 1991; 111:84–91.

36. Schmidt-Erfurth U, Miller JW, Sickenberg M. . Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a retreatments in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117:1177–87.
37. Sickenberg M, Schmidt-Erfurth U, Miller JW. . A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch Ophthalmol. 2000; 118:327–36.

Go to : 
 | Figure 1.Microscopic examination of the neovascularized area in the cornea after photodynamic therapy (PDT). Initially induced corneal neovascularization was observed before performing PDT (A, B, C). One week after PDT, the neovascularized area of the cornea was smaller in the 150 J/cm2 group (E, F) than in the 50 J/cm2 group (D). The same finding was observed in the corneas two weeks after PDT (G, H, I). The neovascularized area was smaller in the 150 J/cm2 group (H) than in the 50 J/cm2 group (G), and the smallest level of neovascularization was observed in the retreated group (I). |
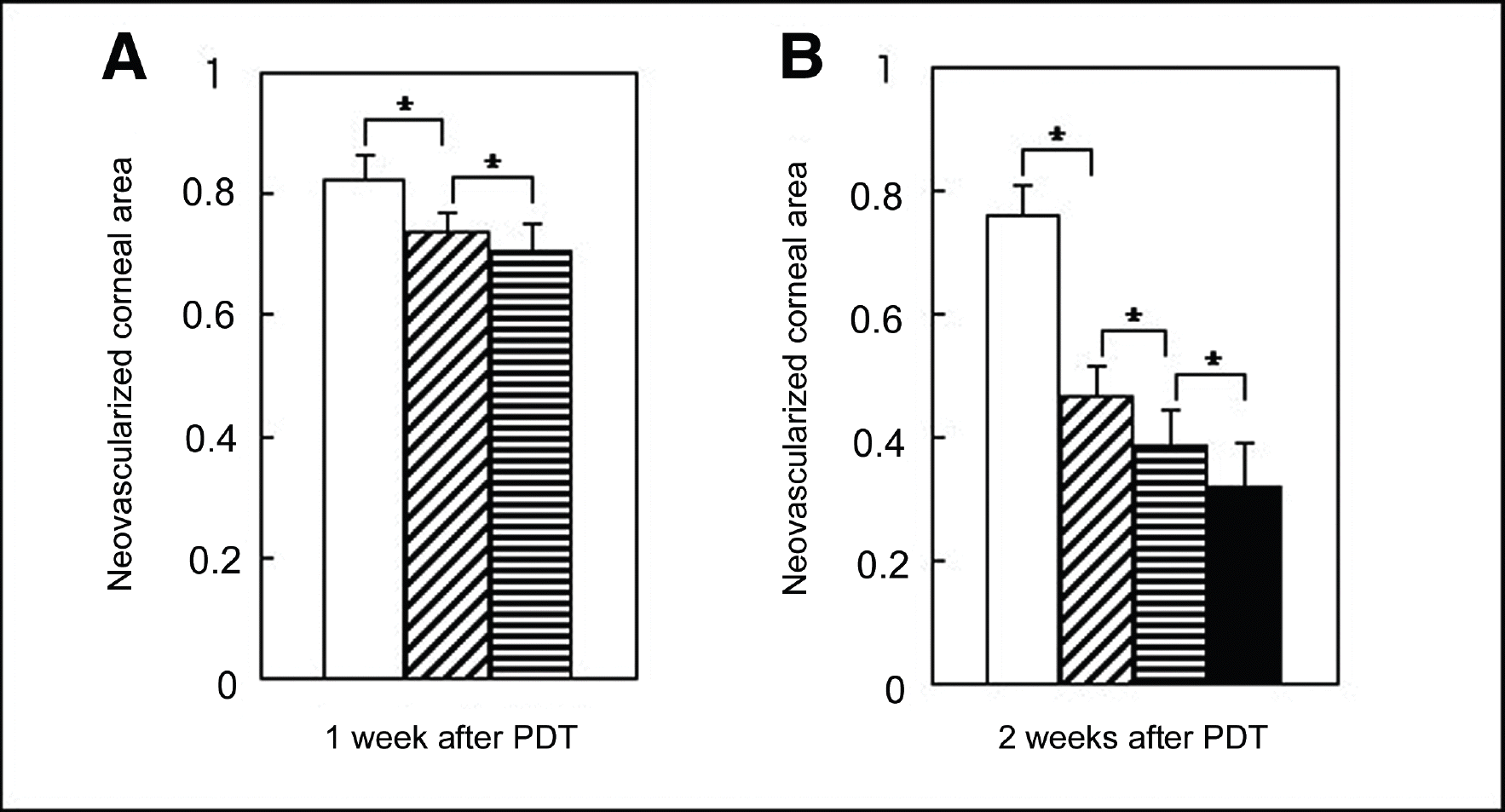 | Figure 2.Comparison of the mean neovascular area in each group after PDT. The corneal neovascular area was standardized to the neovascularized corneal area before performing PDT. (A) One week after PDT, the area of neovascularization was significantly lower in the 50 J/cm2 group ( ) than in the control group ( ) and neovascularization was much lower in the 150 J/cm2 group ( ) than in the 50 J/cm2 group. B. Two weeks after PDT, the area of neovascularization was significantly decreased by the PDT, and the lowest level was observed in the retreated group ( ). (* P<0.05) |
 | Figure 3.Light microscopic examination of a cornea with hematoxylin-eosin staining. New vessels in the cornea were induced experimentally in each group, followed by PDT. New stromal vessels in the cornea could be seen before performing PDT (A, B, C). One week after PDT, the new vessels of the corneal stroma were more regressed in the 150 J/cm2 group (E, F) than in the 50 J/cm2 group (D). The same finding was observed in the 2 -week PDT sections. The new vessels of the corneal stroma were more regressed in the 150 J/cm2 group (H-a) than in the 50 J/cm2 group (G), and they were markedly regressed in the retreated group (arrows, I-a, I-b) compared with the 150 J/cm2 group (arrows, H-a, H-b). H-b and I-b show the magnification view of the boxed area of H-a and I-a, respectively. |
 | Figure 4.Electron microphotographs showing endothelial cellular changes in the new corneal vessels after PDT. (A) Before performing PDT, new vessels in the corneal stroma with a normal vascular endothelial cell structure (nl), vessel wall lining (w) and discoid red blood cells (rbc) were noted. (B) and (C) One week after PDT, condensed chromatin (arrow head) was observed adjacent to the nuclear envelope and an electron dense clump (arrow) was observed. There was no vessel wall lining (dotted arrow). The cytoplasm was vacuolized (v), and there were irregular red blood cells (i-rbc) suggesting thrombosis. (D) Two weeks after PDT, the nucleus of a vascular endothelial cell was fragmented (open arrow). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download