Abstract
Purpose
To evaluate the expression of chemokine and chemokine receptors in the aqueous humor (AH) of patients with recurrent anterior uveitis (AU) in terms of HLA-B-27-association.
Methods
Patients with endogenous uveitis were recruited from the uveitis clinic at Seoul National University Hospital. AH and peripheral blood (PB) were obtained from each patient. The expression of chemokine receptors in T-cells from AH and PB was measured using flow cytometric analysis. Interleukin (IL)-8, interferon-γ inducible protein (IP)-10, and regulated-on-expression, normal T-cell expressed and secreted (RANTES) levels of PB and AH were measured. The expression of chemokine receptor and chemokine levels in PB and AH were compared between HLA-B27-associated AU and idiopathic AU patients.
Results
Seventeen patients with HLA-B27-associated AU, 14 patients with idiopathic AU and 5 healthy controls were included in this study. IL-8 and IP-10 levels of AH were shown to be increased more than in PB, and intraocular concentrations of IL-8 and IP-10 were higher in patients with HLA-B27-associated AU than in idiopathic AU patients. RANTES levels in AH were significantly lower than in PB for all groups. In all groups, the expression of chemokine receptor in AH increased more than in PB.
Go to : 
References
1. de Smet M, Dayan M. Prospective determination of T cell responses to S-antigen in Behçet’s disease patients and controls. Invest Ophthalmol Vis Sci. 2000; 41:3480–4.
2. Brewerton DA, Caffrey M, Nicholls A. . Acute anterior uveitis and HL-A 27. Lancet. 1973; 2:994–6.

3. Ali A, Samson M. Seronegative spondyloarthropathies and the eye. Curr Opin Ophthalmol. 2007; 18:476–80.

4. Power WJ, Rodriguez A, Pedroza-Seres M, Foster CS. Outcomes in anterior uveitis associated with the HLA-B27 haplotype. Ophthalmology. 1998; 105:1646–51.

5. Otasevic L, Zlatanovic G, Stanojevic-Paovic A. . Helicobacter pylori: an underestimated factor in acute anterior uveitis and spondyloarthropathy? Ophthalmologica. 2007; 221:6–13.
6. Reveille JD, Arnett FC. Spondyloarthritis: update on pathogenesis and management. Am J Med. 2005; 118:592–603.

7. Kim TH, Uhm WS, Inman RD. Pathogenesis of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 2005; 17:400–5.

9. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000; 12:121–7.
10. Bonecchi R, Bianchi G, Bordignon PP. . Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998; 187:129–34.

11. Sallusto F, Lenig D, MacKay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998; 187:875–83.

12. Klok AM, Luyendijk L, Zaal MJ. . Elevated serum IL-8 levels are associated with disease activity in idiopathic intermediate uveitis. Br J Ophthalmol. 1998; 82:871–4.

13. Bloch-Michel E, Nussenblatt R. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987; 103:234–5.

14. Rossi D, Zlotinik A. The biology of chemokines and their receptors. Ann Rev Immunol. 2000; 18:217–42.

15. Howard O, Dong H, Su S. . Autoantigens signal through chemokine receptors: uveitis antigens induce CXCR3- and CXCR5-expressing lymphocytes and immature dendritic cells to migrate. Blood. 2005; 105:4207–14.

16. Wallace GR, Curnow SJ, Wloka K. . The role of chemokines and their receptors in ocular disease. Prog Retin Eye Res. 2004; 23:435–48.

17. Fang IM, Yang CH, Lin CP. . Expression of chemokine and receptors in Lewis rats with experimental autoimmune anterior uveitis. Exp Eye Res. 2004; 78:1043–55.

18. Kaburaki T, Fujino Y, Kawashima H. . Plasma and whole-blood chemokine levels in patients with Behcet’s disease. Graefes Arch Clin Exp Ophthalmol. 2003; 241:353–8.

19. Abu El-Asrar AM, Struyf S, Descamps FJ. . Chemokines and gelatinases in the aqueous humor of patients with active uveitis. Am J Ophthalmol. 2004; 138:401–11.

21. Frassanito MA, Dammacco R, Fusaro T. . Combined cyclosporine-A/prednisone therapy of patients with active uveitis suppresses IFN-γ production and the function of dendritic cell. Clin Exp Immunol. 2003; 133:233–9.
22. Yang CH, Fang IM, Lin CP. . Effects of the NF-kappa B inhibitor pyrrolidine dithiocarbamate on experimentally induced autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2005; 46:1339–47.
23. Crane IJ, Mckillop-Smith S, Wallace CA. . Expression of the chemokines MIP-1α, MCP-1, and RANTES in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2001; 42:1547–52.
24. Ohta K, Yamagami S, Wiggert B. . Chemokine gene expression in iris-ciliary body during experimental autoimmune uveoretinitis. Curr Eye Res. 2002; 24:451–7.

25. Keino H, Takeuchi M, Kezuka T. . Chemokine and chemokine receptor expression during experimental autoimmune uveoretinitis in mice. Graefes Arch Clin Exp Ophthalmol. 2003; 241:111–5.

26. Onal S, Kazokoglu H, Incili B. Prevalence and levels of serum antibodies to gram negative microorganisms in Turkish patients with HLA-B27 positive acute anterior uveitis and controls. Ocul Immunol Inflamm. 2006; 14:29309.

27. Gür-Toy G, Lenk N, Yalcin B. . Serum interleukin-8 as a serologic marker of activity in Behcet's disease. Int J Dermatol. 2005; 44:657–60.

28. Murphy P. The molecular biology of leukocyte chemoattractant receptors, Annu Rev Immunol. 1994; 12:593–633.
29. Sauty A, Colvin R, Wagner L. . CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11). J Immunol. 2001; 167:7084–93.
30. Hess C, Means T, Autissier P. . IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood. 2004; 104:3463–71.

31. Hattar K, Fink L, Fietzner K. . Cell density regulates neutrophil IL-8 synthesis: role of IL-1 receptor antagonist and soluble TNF receptors. J Immunol. 2001; 166:6287–93.

32. Yu HG, Lee DS, Seo JM. . The number of CD8+ T cells and NKT cells increases in the aqueous humor of patients with Behçet’s uveitis. Clin Exp Immunol. 2004; 137:437–43.

33. Ahn JK, Chung H, Lee DS. . CD8brightCD56+ T cells are cytotoxic effectors in patients with active Behçet's uveitis. J Immunol. 2005; 175:6133–42.
Go to : 
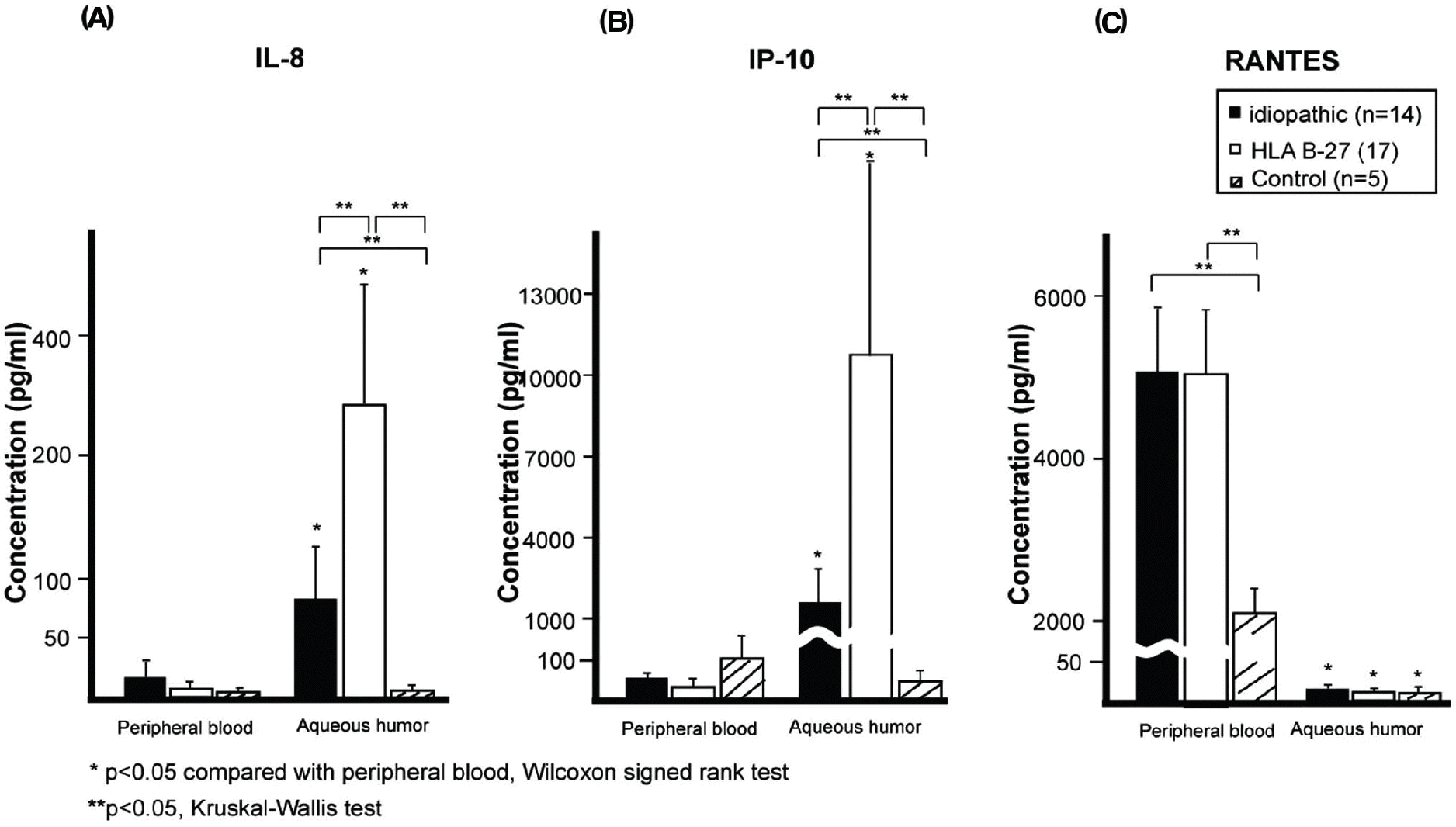 | Figure 1.Chemokine levels in the peripheral blood (PB) and the aqueous humor (AH) from patients with idiopathic and HLA-B27-associated anterior uveitis (AU). (A, B) Intraocular IL-8 and IP-10 levels in patient groups were significantly increased than those of PB. IL-8 and IP-10 levels in the aqueous humor of patients with HLA B-27-associated AU were higher than in patients with idiopathic AU ( p=0.001, Kruskal-Wallis test). (C) RANTES levels in AH were significantly lower than those in PB in all groups and were not different between the groups. RANTES levels in PB of patient groups were significantly higher than those of healthy controls, but RANTES levels in PB were not different between the patient groups. |
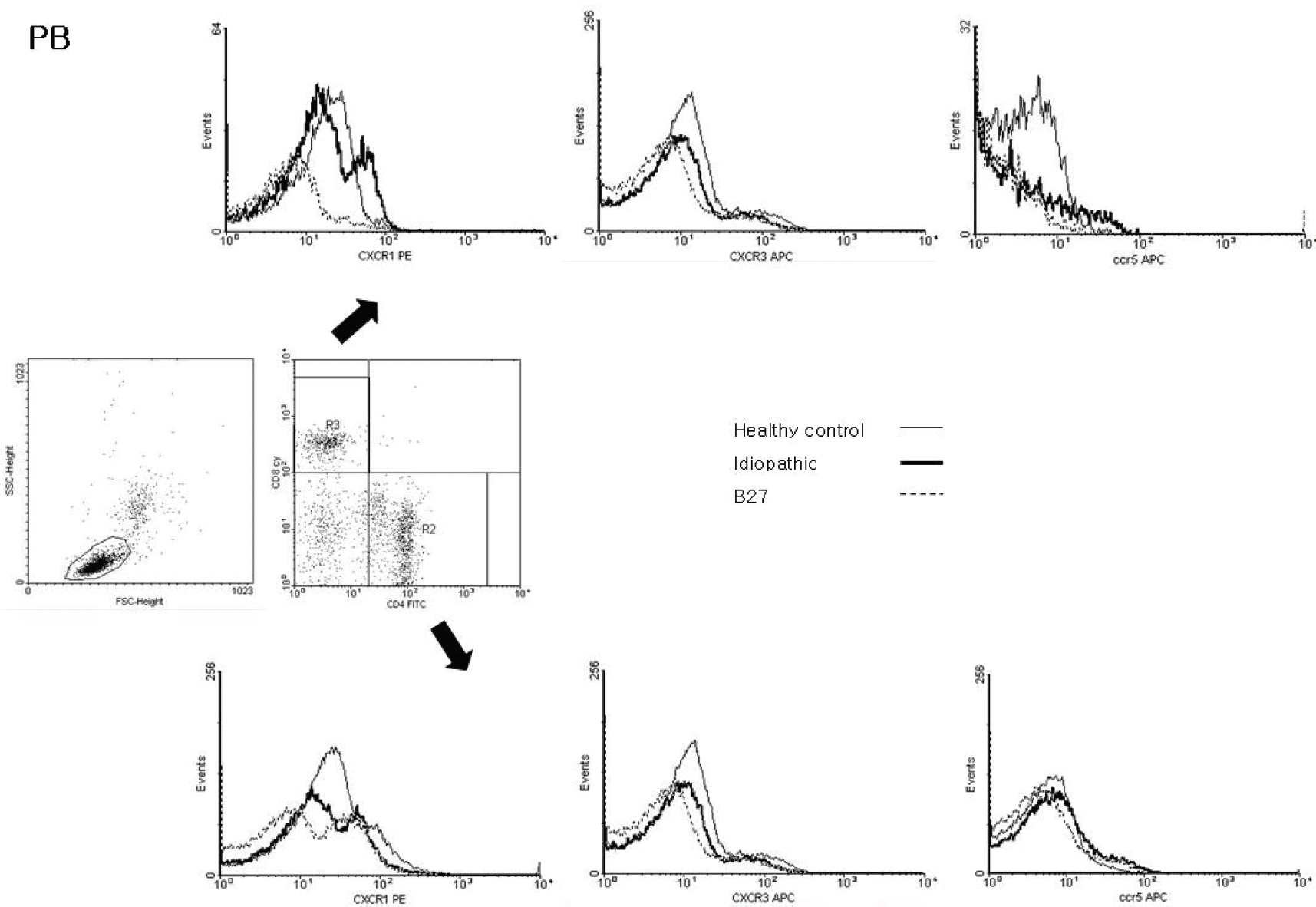 | Figure 2.Representative dot-plot diagrams and histograms which show the expression of CXCR1, CXCR3, and CCR5 in the peripheral blood cells from patients with healthy controls, idiopathic and HLA-B27-associated anterior uveitis. (middle) The region where lymphocytes were accumulated was gated according to forward scatter and side scatter. The percentage of CD4+ and CD8+ T cells subsets among the lymphocytes were shown. The expression of CXCR1, CXCR3 and CCR5 in CD8+ (upper) and CD4+ (lower) T lymphocytes of peripheral blood samples was not different in comparisons among the groups. |
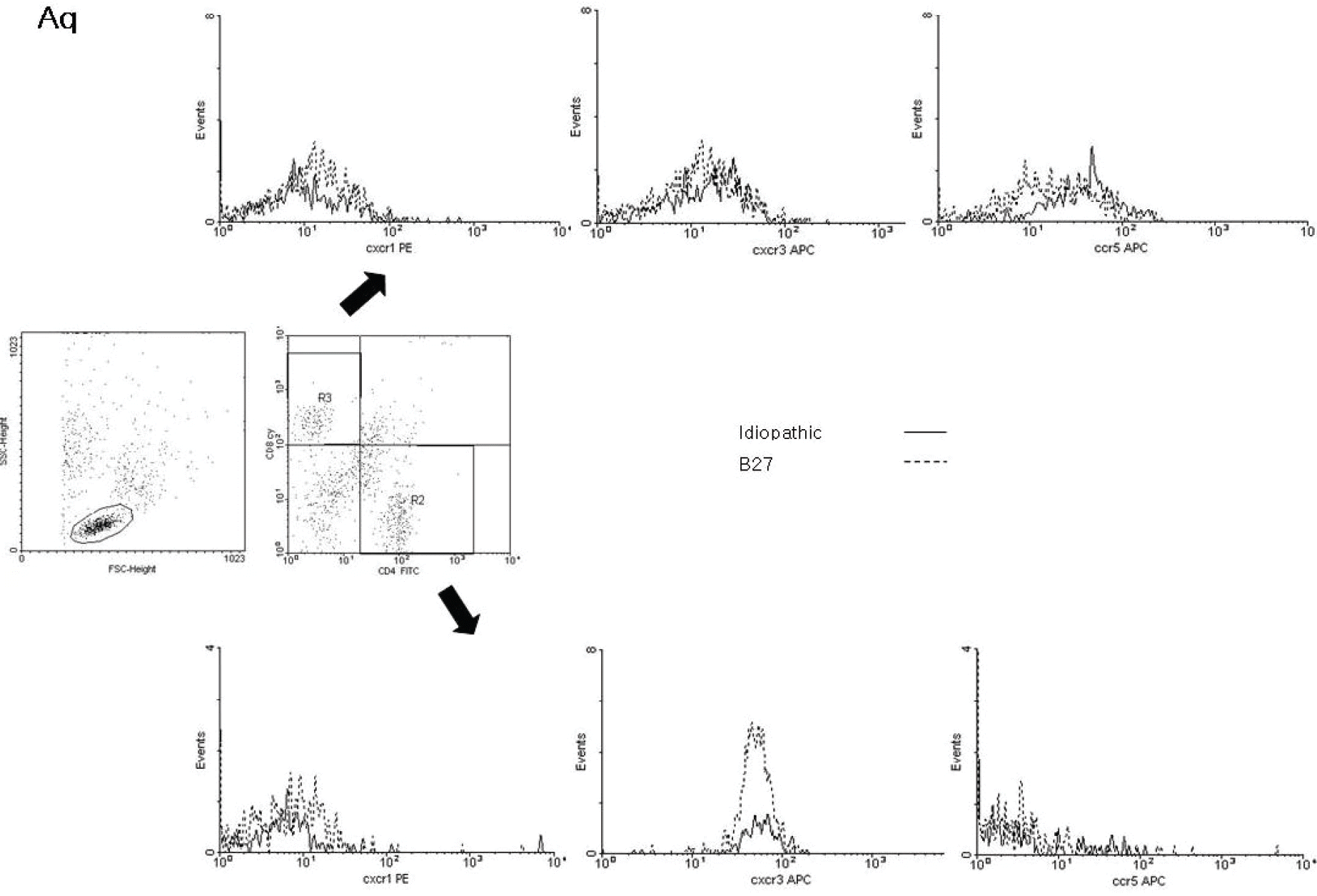 | Figure 3.Representative dot-plot diagrams and histograms which show the expression of CXCR1, CXCR3, and CCR5 in the aqueous humor cells from patients with idiopathic and HLA-B27-associated anterior uveitis. (middle) The region where lymphocytes were accumulated was gated according to forward scatter and side scatter. The percentage of CD4+ and CD8+ T cells subsets among the lymphocytes were shown. The expression of CXCR1, CXCR3 and CCR5 in CD8+ (upper) and CD4+ (lower) T lymphocytes of aqueous humor samples was not different in comparisons among the groups. The surface phenotype of the cells from aqueous humor of healthy controls could not be identified because of the low cell count. |
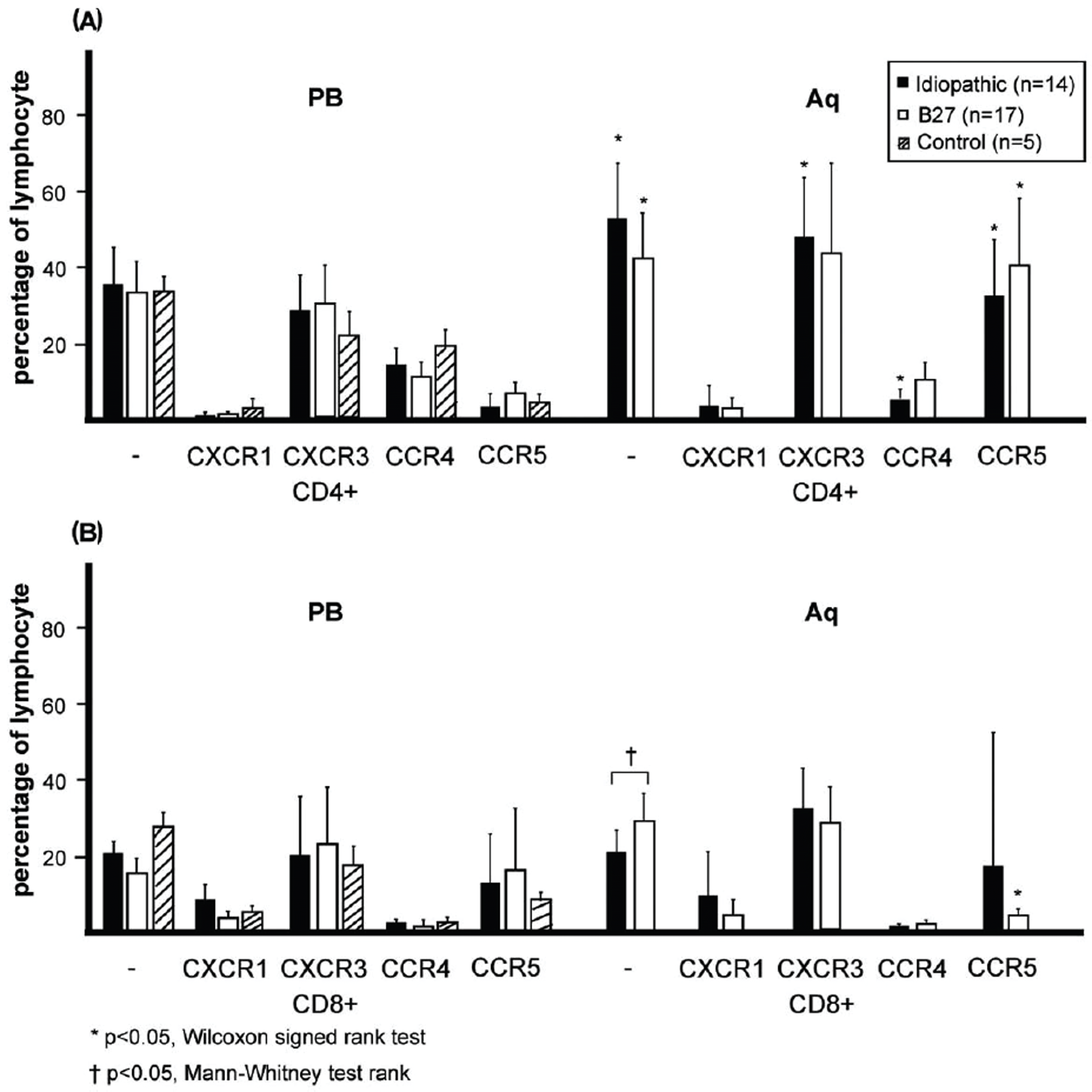 | Figure 4.The expression of chemokine receptors in patients with idiopathic and HLA-B27-associated anterior uveitis (AU). In two groups, the CD4+ T cell population in the aqueous humor (AH) was higher than that in the peripheral blood (PB). (A) In patients with idiopathic AU, the percentages of intraocular CD4+CXCR3+ and CD4+CCR5+ T cells increased than those of PB. The percentage of CD4+CCR4+ T cells in AH was lower than that in PB ( p<0.05, Wilcoxon signed rank test). In patients with HLA-B27-associated AU, the percentage of intraocular CD4+CCR5+ T cells increased than that of PB. (B) The percentage of CD8+CCR5+ T cells in AH was lower than that in PB ( p<0.05, Wilcoxon signed rank test). |
Table 1.
Clinical features in patients with idiopathic and HLA-B27-associated anterior uveitis
| Idiopathic anterior uveitis (n=14) | anterior uveitis (n=17) HLA-B27-associated | p-value | |
|---|---|---|---|
| Age (years) | 38.4±17.5 | 34.8±11.3 | 0.73 |
| Sex (M:F) | 6:8 | 7:10 | 0.93* |
| Duration of uveitis (mo) | 2.4±3.2 | 2.2±2.4 | 0.71 |
| Number of patients with topical steroid at sampling | 6 | 12 | 0.12∗ |
| Systemic association | Ankylosing spondylitis: 3 | ||
| Reiter syndrome: 1 | |||
| Juvenile rheumatoid arthritis: 1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download