Abstract
Purpose
To evaluate the short-term effect and safety of intravitreally injected bevacizumab in patients with macular edema (ME) caused by retinal vein occlusion (RVO) and diabetic macular edema (DME).
Methods
We retrospectively evaluated 59 eyes of 51 patients, 29 with ME caused by RVO and 30 with DME, who received intravitreal injection of bevacizumab. Fifty-one consecutive patients (59 eyes) with ME associated with RVO and DME were treated with intravitreal injections of 1.25-2.5 mg (0.05-0.1 ml) of bevacizumab. Ophthalmic evaluation was performed at baseline and at 1, 3, 6 months after each injection. Clinical evidence of toxicity and complications, changes of visual acuity with an ETDRS chart (LogMAR), and central macular thickness (CMT) using optical coherence tomography (OCT), were evaluated.
Results
The follow-up period was 7.3 months (7.3±0.31) and the mean number of injections was 1.2. The baseline mean LogMAR was 1.06±0.53 and mean CMT was 479.6±160.4 µm. At 1, 3 and 6 months, the mean LogMAR was 0.90±0.52, 0.80±0.39 and 0.78±0.39, respectively, and the mean CMT was 316.9±86.7 µm, 281.1±67.4 µm and 278.4±64.6 µm, respectively. No adverse incidents were observed, including cataract, retinal detachment, vitreous hemorrhage, and endophthalmitis, although transient increased intraocular pressure was observed.
Go to : 
References
1. Early Treatment Diabetic Retinopahty Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–806.
2. The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in brach vein occlusion. Am J Ophthalmol. 1984; 98:271–82.
3. Pendergast SD, Hassan TS, Williams GA. . Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloids. Am J Ophthalmol. 2000; 130:178–86.
4. Martidis A, Duker JS, Greenberg PB. . Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

5. Chen SD, Lochhead J, Patel CK, Frith P. Intravitreal triamcinolone acetonide for ischaemic macular oedema caused by branch retinal vein occlusion. Br J Ophthalmol. 2004; 88:154–5.

6. Greenberg PB, Martidis A, Rogers AH. . Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.

7. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998; 82:561–8.

8. Adamis AP, Miller JW, Bernal MT. . Increase vascular endothelia growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994; 118:445–50.
9. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004; 25:581–611.

10. Funatsu H, Yamashita H, Noma H. . Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005; 243:3–8.

11. Macugen Diabetic Retinopathy Study Group. A phase II randomized double-marked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005; 112:1747–57.
12. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–9.

13. Michels S, Rosenfeld PJ, Puliafito CA. . Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005; 112:1035–47.
14. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3:391–400.

15. Kabbinavar F, Hurwitz HI, Fehrenbacher L. Phase II. randomized trial comparing bevacizumab plus fluorouracil(FU)/ leucovorin(LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003; 21:60–65.
16. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26:275–8.

17. Iturralde D, Spaide RF, Meyerle CB. . Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
18. Meyer-Schwickerath R, Pfeiffer A, Blum WF. . Vitreous level of insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in noevascular eye disease: studies in nondiabetic and diabetic subjects. J Clin Invest. 1993; 92:2620–5.
19. Floman N, Zor U. Mechanism of steroid action in ocular inflammation: inhibition of prostaglandin production. Invest Ophthalmol Vis Sci. 1977; 16:69–73.
20. Bandi N, Kompella UB. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur J Pharmacol. 2001; 425:109–16.

21. Gillies MC, Simpson JM, Billson FA. . Safety of an intravitreal injection of Triamcinolone. Arch Ophthalmol. 2004; 122:336–40.

22. Jonas JB, Kreissig I, Degenring RF. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–7.

23. Moshfeghi DM, Kaiser PK, Scott IU. . Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.

24. Islam MS, Vernon SA, Negi A. Intravitreal triamcinolone will cause posterior subcapsular cataract in most eyes with diabetic maculopathy within 2 years. Eye. 2007; 21:321–3.

25. Gottfredottir MS, Stefansson E, Jonasson F. . Retinal vasoconstriction after laser treatment for diabetic macular edema. Am J Ophthalmol. 1993; 115:64–7.
26. McAllister IL, Douglas JP, Constable IJ, Yu DY. Laser induced chorioretinal venous anastomosis for nonischemic central vein occlusion: evaluation of the complications ans their risk factors. Am J Ophthalmol. 1998; 126:219–29.
27. Fekrat S, Goldberg MF, Finkelstein D. Laser induced chorioretinal venous anastomosis for nonischemic central or branch retinal vein occlusion. Arch Ophthalmol. 1998; 116:43–52.
28. Browning DJ, Antoszyka N, McAllister IL. Laser Chorioretinal venous anastomosis for nonischemic central retinal vein occlusion. Ophthalmology. 1998; 105:670–9.
29. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1:27–31.

30. Michaelson IC. Vascular morphogenesis in the retina of the cat. J Anat. 1948; 82:167–174.
31. Patz A. Studies on retinal neovascularization. Invest Ophthalmol Vis Sci. 1980; 19:1133–8.
32. Kabbinavar F, Hurwitz HI, Fehrenbacher L. . Phase II randomized trial comparing bevacizumab plus fluorouracil (FU) / leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003; 21:60–65.
33. Nguyen QD, Shah S, Tatlipinar S. . Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol. 2005; 89:1368–70.

34. Yamamoto I, Rogers AH, Reichel ER. . Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2007; 91:157–60.

35. Gomi F, Nishida K, Oshima Y. . Intravitreal Bevacizumab for Idiopathic Choroidal Neovascularization After Previous Injection With Posterior Subtenon Triamcinolone. Am J Ophthalmol. 2007; 143:507–10.

36. Tong JP, Chan WM, Liu DT. . Aqueous humor levels of vascular endothelial growth factor and pigment epithelium- derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141:456–62.
37. Matsuoka M, Ogata N, Otsuji T. . Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88:809–15.

38. Avery RL, Pieramici DJ, Rabena MD. . Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–72.

39. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:270–1.

40. Costa RA, Jorge R, Calucci D. . Intravitreal bevacizumab for choroidal neovascularization caused by AMD: results of a phase I dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–78.
41. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–9.

42. Iturralde D, Spaide RF, Meyerle CB. . Intravitreal bevacizumab (Avastin) treatment of macular edema in central vein occlusion: a short-term study. Retina. 2006; 26:279–84.
43. Spandau UH, Ihloff AK, Jonas JB. Intravitreal bevacizumab treatment of macular edema due to central retinal vein occlusion. Acta Ophthalmol Scand. 2006; 84:555–6.
44. Arevalo JF. . Primary intravitreal bevacizumab (Avastin) for diabetic macular edema. Ophthalmology. 2007; 114:743–50.

45. Pieramici DJ, Avery RL, Castellarin AA. . Case of anterior uveitis after intravitreal injection of bevacizumab. Retina. 2006; 26:841–2.

46. Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization secondary to age related macular degeneration. Br J Ophthalmol. 2006; 90:1207–8.
Go to : 
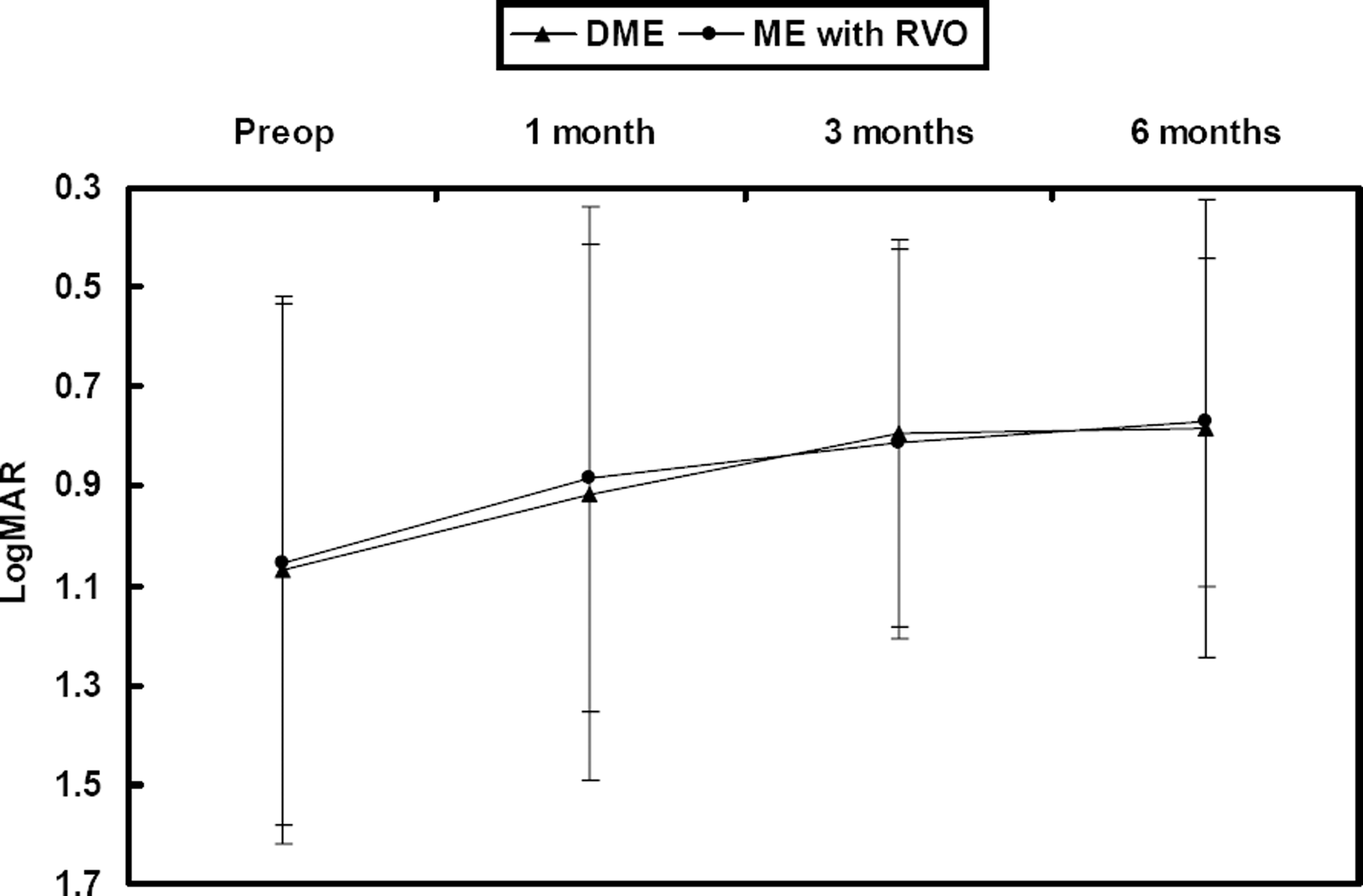 | Figure 1.Changes in LogMAR after intravitreal bevacizumab injections, according to macular edema etiology; DME=diabetic macular edema; ME=macular edema; RVO=retinal vein occlusion. |
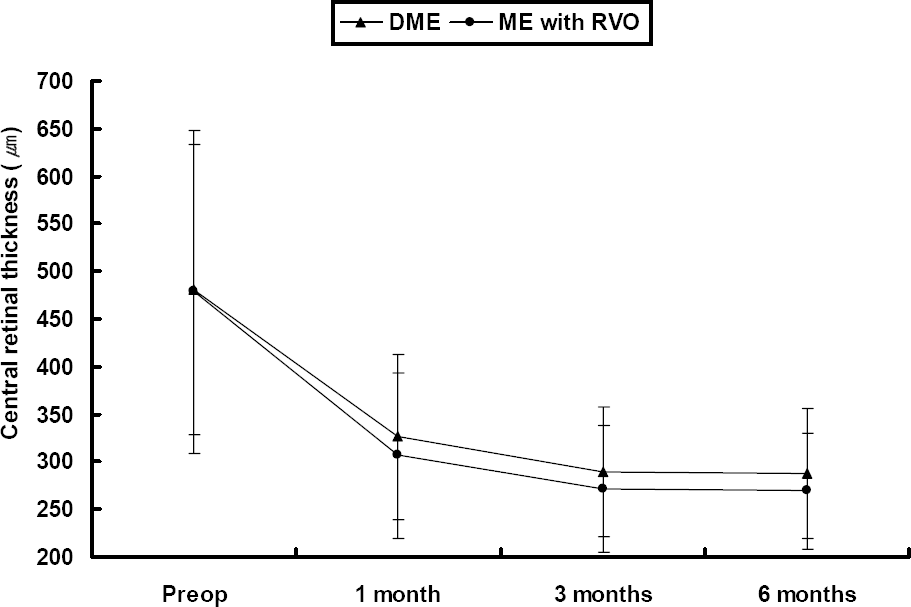 | Figure 2.Changes of central macular thickness with optical coherence tomography (OCT) after intravitreal bevacizumab injections. DME=diabetic macular edema; ME=macular edema; RVO= retinal vein occlusion. |
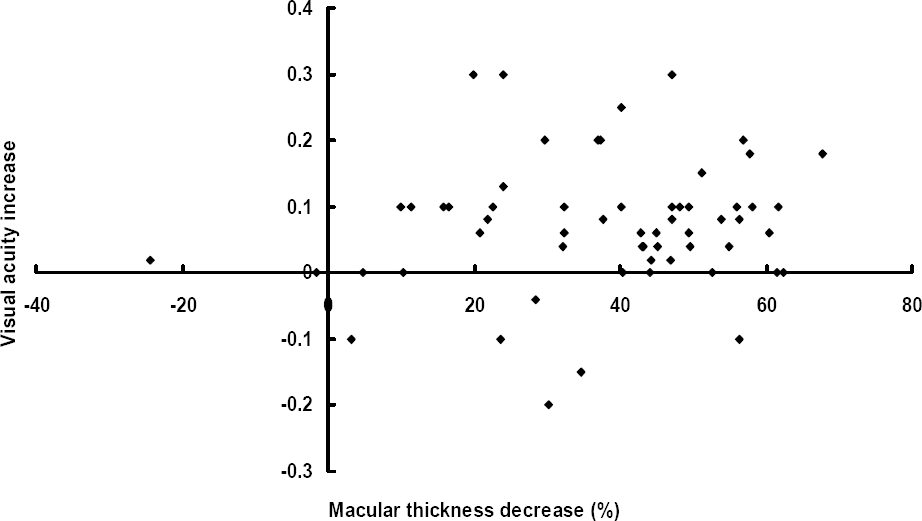 | Figure 3.The relationship between visual acuity increase and macular thickness decrease (%) at 6 months after intravitreal bevacizumab injections. |
Table 1.
Patients’ characteristics
| Variable | Data |
|---|---|
| Age (years) | |
| Mean±SD* | 59.0±7.2 |
| Range | 30-79 |
| Total (eyes, %) | |
| Male | 32 (54.2%) |
| Female | 27 (45.8%) |
| Unerlying disease (%) | |
| Diabetic retinopathy, Proliferative | 17 (28.8%) |
| Diabetic retinopathy, Non-Proliferative | 13 (22.0%) |
| CRVO† | 17 (28.8%) |
| BRVO‡ | 12 (20.3%) |
| Follow up period (months) | |
| Mean±SD* | 7.3±0.31 |
| Range | 6-8 |
| Preoperative BCVA§ (LogMAR#, mean±SD*) | 1.06±0.53 |
| Preoperative central macular thickness (µm, mean±SD*) | 479.6±160.4 |
Table 2.
The numbers of eyes, mean age, mean follow-up time, BCVA*, central macular thickness before intravitreal bevacizumab injection
| DME | RVO | BRVO | CRVO | |
|---|---|---|---|---|
| Age (years) | 57.9 | 61.5 | 65.4 | 57.5 |
| Total (eyes) | 30 | 29 | 12 | 17 |
| Male | 16 | 16 | 4 | 12 |
| Female | 14 | 13 | 8 | 5 |
| Mean follow up time(months) | 7.1 | 7.4 | 7.5 | 7.3 |
| Preoperative BCVA* | ||||
| (LogMAR†,mean±SD§)/ | 1.06±0.53 / | 1.07±0.55 / | 0.62±0.41/ | 1.38±0.4 / |
| CMT‡(µm, mean±SD§) | 480.7±153.2 | 478.4±170.2 | 406.6±96.9 | 529.2±193.9 |
Table 3.
Changes in visual acuity and central retinal thickness in macular edema caused by diabetic macular edema(DME)
| Baseline | 1 month | 3 months | 6 months | |
|---|---|---|---|---|
| BCVA* | 1.06±0.53 | 0.88±0.47 | 0.81±0.40 | 0.77±0.33 |
| (LogMAR†, mean±SD#) | (P=0.006)§ | (P<0.001)§ | (P<0.001)§ | |
| CMT‡ | 480.7±153.2 | 326.5±86.9 | 290±68.2 | 287.4±67.7 |
| (µm, mean±SD#) | (P<0.001)§ | (P<0.001)§ | (P<0.001)§ |
Table 4.
Changes in visual acuity and central retinal thickness in macular edema caused by retinal vein occlusion (RVO)
| Baseline | 1 month | 3 months | 6 months | |
|---|---|---|---|---|
| BCVA* | 1.07±0.55 | 0.91±0.57 | 0.79±0.39 | 0.78±0.46 |
| (LogMAR†, mean±SD#) | (P=0.002)§ | (P=0.006)§ | (P=0.001)§ | |
| CMT‡ | 478.4±170.2 | 307.1±86.8 | 271.9±66.4 | 269.1±60.9 |
| (µm, mean±SD#) | (P<0.001)§ | (P<0.001)§ | (P<0.001)§ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download