Abstract
Purpose
To evaluate the involvement of apoptosis in N-methyl-D-aspartate (NMDA)-induced excitotoxicity in the rabbit retina.
Methods
After intravitreal injection of 680 and 2,000 nmoles of NMDA in rabbit eyes, the eyes were enucleated at 2, 16, and 60 hours and 1 and 2 weeks. The apoptotic cell death was determined with TdT-mediated biotin-dUTP nick end labeling (TUNEL) stain, and immunohistochemical stains of Bcl-2, Bax, and caspase-3 were performed.
Results
TUNEL showed increased labeling scattered in the ganglion cell layer and inner nuclear layer from 16 to 60 hours. The number of TUNEL-positive nuclei decreased at 60 hours, and none was observed at 2 hours, 1 week, and 2 weeks. More TUNEL-positive nuclei were seen with injection of 2,000 nmoles compared to 680 nmoles. Bcl-2, Bax, and caspase-3 were seen histologically as early as 2 hours in the ganglion cell layer and inner nuclear layer; there was no stained nuclei with the TUNEL stain. At 2 hours after intravitreal NMDA injection, Bcl-2, Bax, and caspase-3 were also stained in Müller cells.
Go to : 
References
1. Buchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992; 55:605–13.
2. Matini P, Moroni F, Lombardi G. . Ultrastructural and Biochemical studies on the neuroprotective effects of excitatory amino acid antagonists in the ischemic rat retina. Exp Neurol. 1997; 146:419–34.

4. Nakanishi S, Nakajima Y, Masu M. . Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev. 1998; 26:230–5.
5. Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999; 18:765–810.

7. Kageyama T, Ishikawa A, Tamai M. Glutamate elevation in rabbit vitreous during transient ischemia-reperfusion. Jpn J Ophthalmol. 2000; 44:110–4.

8. Dreyer EB, Zurakowski D, Schumer RA. . Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996; 114:299–305.

9. Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-D- aspartate (NMDA) induced apoptosis in rat retina. Invest Opthalmol Vis Sci. 1999; 40:2319–7.
10. Kwong JM, Lam TT. N-methyl-D-aspartate (NMDA) induced apoptosis in adult rabbit retinas. Exp Eye Res. 2000; 71:437–44.
11. Moroni F, Lombardi G, Pellegrini-Faussone S, Moroni F. Photochemically induced lesion of the rat retina: a quantitative model for the evaluation of ischemia induced retinal damage. Vison Res. 1993; 33:1887–91.
12. Kasischke K, Ludolph AC, Riepe MW. NMDA-antagonists increased hypoxic tolerance by preceding chemical hypoxia. Neurosci Lett. 1996; 214:175–8.
13. Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci. 1995; 15:7702–11.

14. Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Glutamate-induced over expression of NMDA receptor messenger RNAs and protein triggered by activation of AMPA/kainate receptors in rat hippocampus following forebrain ischemia. Brain Res. 1994; 659:67–74.
15. Louzada-Junior P, Dias JJ, Santos WF. . Glutamate release in experimental ischemia of the retina an approach using microdialysis. J Neurochem. 1992; 59:358–63.
16. Neal MJ, Cunningham JR, Hutson PH, Hogg J. Effects of ischemia on neurotransmitter release from the isolated retina. J Neurochem. 1994; 62:1025–33.
17. Wahl F, Obrenovitch TP, Hardy AM. . Extracellular glutamate during focal cerebral ischemia in rats: time course and calcium dependency. J Neurochem. 1994; 63:1003–11.
18. Tsubokawa H, Oguro K, Masuzawa T, Kawai N. Ca2+-dependent non-NMDA receptor-mediated synaptic currents in ischemic CA1 hippocampal neurons. J Neurophysiol. 1994; 71:1190–6.
19. Ferreira IL, Duarte CB, Carvalho AP. Ca2+ influx through glutamate receptor-associated channels in retina cells correlates with neuronal cell death. Eur J Pharmacol. 1996; 302:153–62.
20. Dugan LL, Sensi SL, Canzoniero LM. . Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995; 15:6377–88.

21. Prigle AK, Iannotti F, Wilde GJ. . Neuroprotection by both NMDA and non-NMDA receptor antagonists in vitro ischemia. Brain Res. 1997; 755:36–46.
22. Lee SJ, Jung CS, Bai SH. The effects of N-methyl-D-aspartic acid and antagonism by Kyurenic acid on neurons in the catfish retina. J Korean Ophthalmol Soc. 1998; 39:2303–12.
23. Rosl F. A simple and rapid method for detection of apoptosis in human cells. Nucl Acids Res. 1992; 20:5243.
24. Gavrieli Y, Sherman Y, Ben-Sasson . Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992; 119:493–501.

25. Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998; 8:324–30.

26. Yang E, Zha J, Jockel J. . Bad, a heterodimeric partner for Bcl-X L and Bcl-2, displaces Bax and promotes cell death. Cell. 1995; 80:285–91.
27. O'Connor L, Strasser A, O'Reilly LA. . Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998; 17:384–95.
28. Boise LH, Gonzalez-Garcia M, Postema CE. . BCL-X, a BCL-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993; 74:597–608.

29. Lithgow T, van Driel R, Bertram JF, Strasser A. The protein product of the oncogene Bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994; 5:411–7.
30. Hockenbery DM, Oltvai ZN, Yin XM. . Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993; 75:241–51.

31. Kane DJ, Sarafian TA, Anton R. . Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993; 262:1274–7.

32. Mazel S, Burtrum D, Petrie HT. Regulation of cell division cycle progression by Bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996; 183:2219–26.

33. Harris MH, Thompson CB. The role of Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000; 7:1182–91.
34. Oltvai Z, Milliman C, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–19.
35. Bonfanti L, Strettoi E, Chierzi S. . Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing Bcl-2. J Neurosci. 1996; 16:4186–94.

36. Porciatti V, Pizzorusso T, Cenni MC, Maffei L. The visual response of retinal ganglion cells is not altered by optic nerve transection in transgenic mice overexpressing Bcl-2. Proc Natl Acad Aci U S A. 1996; 93:14955–9.

37. Khaled AR, Kim K, Hofmeister R. . Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U.S.A. 1999; 96:14476–81.

38. Ogden TE. Ryan SJ, editor. Glia of the retina. Retina. 1994. 2nd ed. St. Louis: The C.V. Mosby Company;v. 1:p. chap. 5.
39. Ehinger B. Glial and neuronal uptake of GABA, glutamic acid, glutamine and glutathione in the rabbit retina. Exp Eye Res. 1977; 25:221–34.

40. Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990; 348:443–6.

41. Pow DV, Robinson SR. Glutamate in some retinal neurons is derived solely from glia. Neuroscience. 1994; 60:355–66.

42. Chen ST, Gentleman SM, Garey LJ, Jen LS. Distribution of beta-amyloid precursor and B-cell lymphoma protooncogene proteins in the rat retina after optic nerve transection or vascular lesion. J Neuropathol Exp Neurol. 1996; 55:1073–82.
43. Park KH, Kim DM. Apoptosis of retinal ganglion cell after ischemia-reperfusion injury of optic nerve in rabbits. J Korean Ophthalmol Soc. 1998; 39:2687–700.
46. Faraco PR, Ledgerwood EC, Vandenabeele P. . Tumor necrosis factor induces distinct patterns of caspase activation in WEHI-164 cells associated with apoptosis or necrosis depending on cell cycle stage. Biochem Biophys Res Commun. 1999; 261:385–92.

47. Tezel G, Wax MB. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest Ophthalmol Vis Sci. 1999; 40:2660–7.
Go to : 
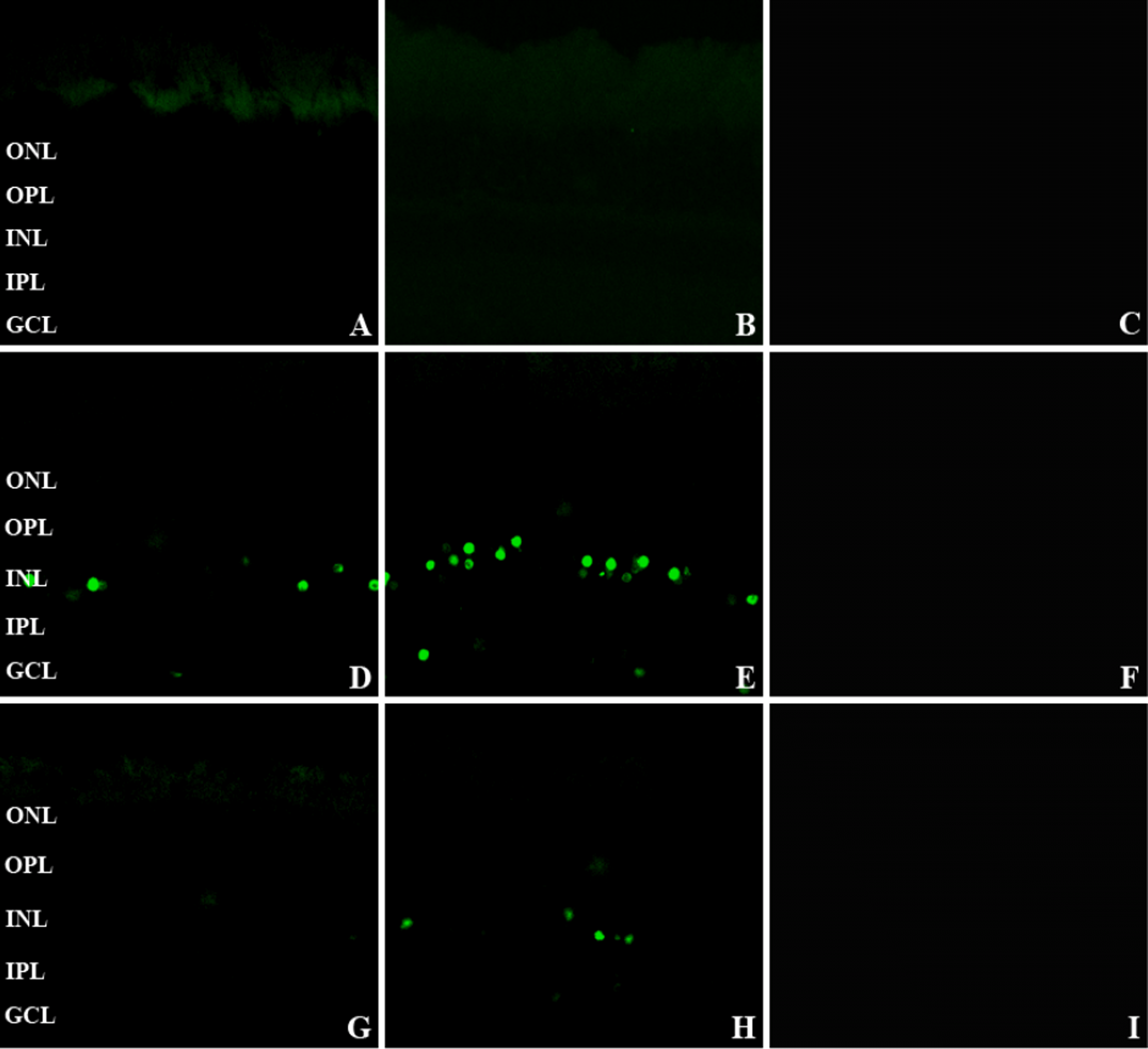 | Figure 1.TUNEL stain of the retinas at 2 (A), 16 (D), 60 (G) hours after 680 nmoles of intravitreal NMDA injection and at 2 (B), 16 (E), 60 (H) hours after 2,000 nmoles of intravitreal NMDA injection and at 2 (C), 16 (F), 60 (I) hours after PBS injection (×400). A, B: No noticeable labeled nuclei are seen in the ganglion cell layer (GCL) and the inner nuclear layer (INL). (D, E, H) Scattered and intensely labeled nuclei are seen in the GCL and INL. G: No labeled nuclei are seen at 60 hours after 680 nmoles of NMDA injection. (C, F, I) No noticeable nuclei are stained after intravitreal PBS injection. GCL=ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPL= outer plexiform layer; ONL=outer nuclear layer. |
 | Figure 2.Bcl-2 immunohistochemical staining of the retinas at 2 (A), 16 (C), 60 (E) hours after 680 nmoles of intravitreal NMDA injection and at 2 (B), 16 (D), 60 (F) hours after 2,000 nmoles of intravitreal NMDA injection (×400). A, B: Bcl-2 are stained in the ganglion cell layer and Müller cell (arrow). C, D, E, F: Bcl-2 are stained strongly in the GCL and INL. GCL=ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPL= outer plexiform layer; ONL=outer nuclear layer. |
 | Figure 3.Bax immunohistochemical staining of the retinas at 2 (A), 16 (C), 60 (E) hours after 680 nmoles of intravitreal NMDA injection and at 2 (B), 16 (D), 60 (F) hours after 2,000 nmoles of intravitreal NMDA injection (×400). (A) Bax are stained in the ganglion cell layer and Müller cell (arrow). (B, C, D, E, F) Bax are stained in the GCL and INL. GCL=ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPL=outer plexiform layer; ONL=outer nuclear layer. |
 | Figure 4.Caspase-3 immunohistochemical staining of retinas at 2 (A), 16 (C), 60 (E) hours after 680 nmoles of intravitreal NMDA injection and at 2 (B), 16 (D), 60 (F) hours after 2,000 nmoles of intravitreal NMDA injection (×400). (A) Caspase-3 are stained in the ganglion cell and Müller cell (arrow). (B) Caspase-3 are stained in the ganglion cell layer. (C, D, E, F) Caspase-3 are stained strongly in the GCL and INL. GCL=ganglion cell layer; IPL=inner plexiform layer; INL=inner nuclear layer; OPL=outer plexiform layer; ONL=outer nuclear layer. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download