Abstract
Purpose
To investigate the clinical effects and side effects of Tacrolimus for ocular Behçet's disease.
Methods
Eight patients (male: 5, age: 35±7.5 (25-48) years) with Behçet's uveitis, refractory posterior uveitis unresponsive to combination treatment with cyclosporine, azathioprine, and oral steroid or significant cyclosporine-related adverse effects were recruited prospectively, and cyclosporine was switched to Tacrolimus. Visual acuity, degree of anterior chamber, and vitreous haze were compared before the conversion and three months after. Oral glucose tolerance, plasma insulin, cholesterol, creatinine, and aspartate (AST)/alanine aminotransferase (ALT) were measured to monitor systemic effects.
Results
Visual acuity did not change significantly after conversion to Tacrolimus. Anterior chamber (seven patients) and vitreous (four patients) inflammation were found before conversion and decreased or disappeared after conversion. Overall plasma levels of glucose, insulin, creatinine, and AST/ALT were similar before and after conversion. However, cholesterol was significantly decreased after conversion (p=0.028). One patient developed diabetes mellitus.
Go to : 
References
1. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimising therapy with steroids and cyclosporin A. Br J Ophthalmol. 1997; 81:1107–12.

2. Choi YK, Kim MH, Yoo JS, Huh W. The rapeutic Effects of Low Dose Triple Agents [Steroid, Cyclosporine and Azathiprine] on Refractory Uveitis. J Korean Ophthalmol Soc. 2000; 41:660–7.
3. Whitcup SM, Salvo EC Jr, Nussenblatt RB. Combined cyclosporine and corticosteroid therapy for sight-threatening uveitis in Behcet's disease. Am J Ophthalmol. 1994; 118:39–45.
4. Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983; 96:275–82.

5. Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporin A therapy in treating chronic, noninfectious uveitis. Ophthalmology. 1996; 103:365–73.

6. Mochizuki M, Masuda K, Sakane T, et al. A clinical trial of FK506 in refractory uveitis. Am J Ophthalmol. 1993; 115:763–9.

7. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot. 1987; 40:1249–55.

8. Jensik SC. Tacrolimus (FK 506) in kidney transplantation: three-year survival results of the US multicenter, randomized, comparative trial. FK 506 Kidney Transplant Study Group. Transplant Proc. 1998; 30:1216–8.
9. Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994; 57:848–51.
10. Aqel BA, Machicao V, Rosser B, et al. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004; 38:805–9.

11. Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989; 143:718–26.
12. Peters DH, Fitton A, Plosker GL, Faulds D. Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs. 1993; 46:746–94.
13. Ligtenberg G, Hene RJ, Blankestijn PJ, Koomans HA. Cardiovascular risk factors in renal transplant patients: cyclosporin A versus tacrolimus. J Am Soc Nephrol. 2001; 12:368–73.
14. Textor SC, Wiesner R, Wilson DJ, et al. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993; 55:1332–9.

15. O'grady JG, Hardy P, Burroughs AK, Elbourne D. The UK and Ireland Liver Transplant Study Group. Randomized Controlled Trial of Tacrolimus Versus Microemulsified Cyclosporin (TMC) in Liver Transplantation: Poststudy Surveillance to 3 Years. Am J Transplant. 2007; 7:137–41.
16. Vincenti F, Jensik SC, Filo RS, et al. A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: evidence for improved allograft survival at five years. Transplantation. 2002; 73:775–82.
17. Kilmartin DJ, Forrester JV, Dick AD. Tacrolimus (FK506) in failed cyclosporin A therapy in endogenous posterior uveitis. Ocul Immunol Inflamm. 1998; 6:101–9.

18. Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the treatment of posterior uveitis refractory to cyclosporine. Ophthalmology. 1999; 106:723–8.

19. Murphy CC, Greiner K, Plskova J, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol. 2005; 123:634–41.

20. Kim HC, Hwang EA, Han SY, et al. Primary immunosuppression with tacrolimus in kidney transplantation: three-year follow-up in a single center. Transplant Proc. 2004; 36:2082–3.

21. Pirsch JD, Miller J, Deierhoi MH, et al. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997; 63:977–83.
22. Cho YM, Park KS, Jung HS, et al. High incidence of tacrolimus-associated posttransplantation diabetes in the Korean renal allograft recipients according to American Diabetes Association criteria. Diabetes Care. 2003; 26:1123–8.

23. Ozdal PC, Ortac S, Taskintuna I, Firat E. Long-term therapy with low dose cyclosporin A in ocular Behcet's disease. Doc Ophthalmol. 2002; 105:301–12.
24. International Study Group for Behcet's disease. Criteria for diagnosis of Behcet's disease. Lancet. 1990; 335:1078–80.
25. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
26. Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia. 2001; 44:929–45.

27. Rohlfing CL, Wiedmeyer HM, Little RR, et al. Defining the relationship between plasma glucose and HbA1c : analysis of glucose profiles and HbA1c in the Diabetes Control and Complication Trial. Diabetes Care. 2002; 25:275–8.
28. Harper SL, Chorich LJ III, Foster CS. Diagnosis of uveitis. In : Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. Philadelphia: Saunders;2002. chap. 6.
29. Nussenblatt RB, Palestine AG, Chan CC, et al. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985; 92:467–71.
31. Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995; 17:584–91.

32. Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002; 13:2962–8.

33. Aisa Y, Mori T, Nakazato T, Shimizu T, et al. Effects of immunosuppressive agents on magnesium metabolism early after allogeneic hematopoietic stem cell transplantation. Transplantation. 2005; 80:1046–50.

34. Navaneethan SD, Sankarasubbaiyan S, Gross MD, et al. Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc. 2006; 38:1320–2.

35. Tamura K, Fujimura T, Tsutsumi T, et al. Transcriptional inhibition of insulin by FK506 and possible involvement of FK506 binding protein-12 in pancreatic beta-cell. Transplantation. 1995; 59:1606–13.
Go to : 
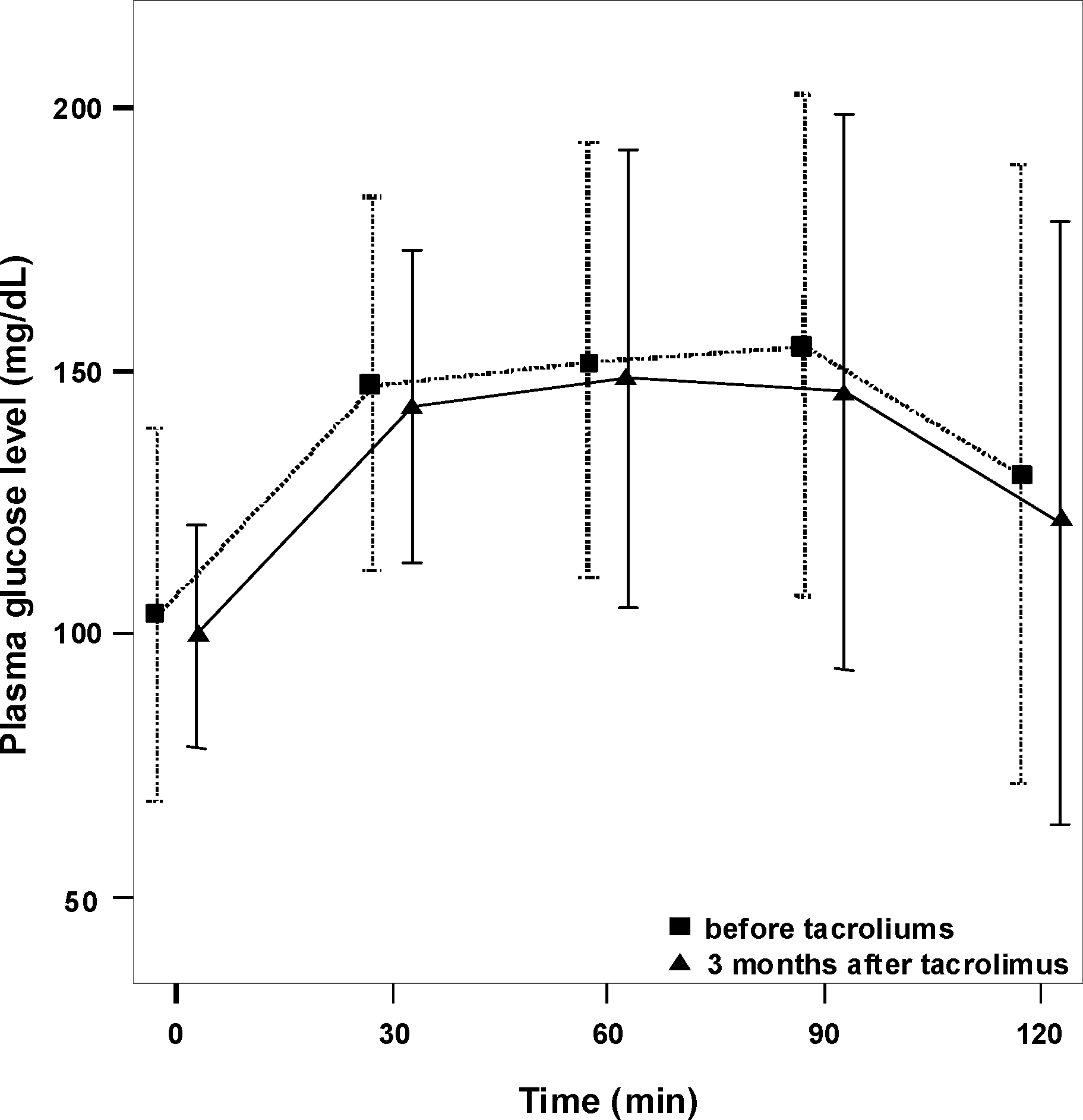 | Figure 1.Comparison of plasma glucose concentrations (oral glucose tolerance test) before and 3 months after conversion from cyclosporine to Tacrolimus. No significant differences are found at all points. |
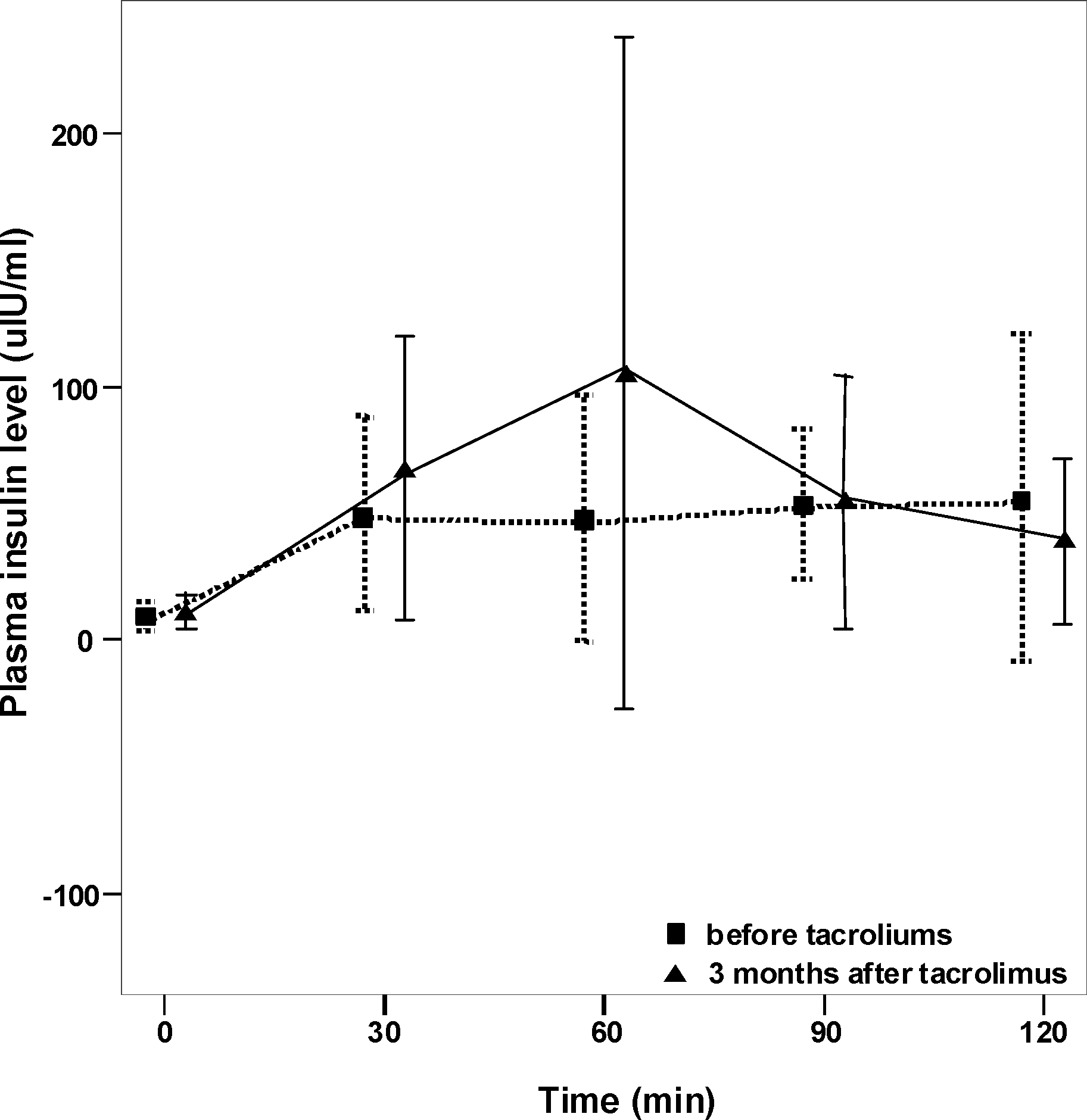 | Figure 2.Comparison of plasma insulin concentrations during oral glucose tolerance test before and 3 months after conversion from cyclosporine to Tacrolimus. No significant differences are found at all points. |
Table 1.
Patient data
Table 2.
Results of treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download