Abstract
Purpose
To evaluate the toxic effect of topical cyclosporine on cultured human corneal epithelium and to investigate the apoptotic response and cellular morphologic changes associated with cyclosporine in vitro.
Methods
Human corneal epithelial cells were exposed to a concentration of cyclosporine A (0.05%) for a period of 3, 5, and 10 minutes. MTT-based calorimetric assay was performed to assess the metabolic activity of cellular proliferation and lactate dehydrogenase (LDH) leakage assay for cytotoxicity. Apoptotic response was evaluated with flow cytometric analysis and fluorescence staining with Annexin V and propiodium iodide. Cellular morphology was evaluated by inverted phase-contrast light microscopy and electron microscopy.
Results
The inhibitory effect of human corneal epithelial cell proliferation and cytotoxicity showed a time-dependent response and had a significant effect when exposed for 10 minutes (P=0.04). The maximun response did not reach the leathal dose (LD)50. Apoptosis was seen in flow cytometry and apoptotic cells were demonstrated in fluorescent micrograph after being treated treating with cyclosporine A (0.05%). Human corneal epithelial cells were more detached from the bottom of the dish and damaged cells show degenerative changes like microvilli disappearance, vacuoles formation, and chromatin of the nuclear remnant condensed along the nuclear periphery.
Go to : 
References
2. Poon AC, Forbes JE, Dart JKG, et al. Systemic cyclosporin A in high risk penetrating keratoplasties: a case-contral study. Br J Ophthalmol. 2001; 85:1464–9.
4. Hill JC. Systemic cyclosporine in high-risk keratoplasty: short versus long term therapy. Ophthalmology. 1994; 101:128–33.
5. Minguez E, Tiestos MT, Cristobal JA, et al. Intraocular absorption of cyclosporin A eyedrops. J Fr Ophthalmol. 1992; 15:263–7.
6. BenEzra D, Maftzir G. Ocular penetration of cyclosporin A: the rabbit eye. Invest Ophthalmol Vis Sci. 1990; 31:1362–6.
8. Chen J, Xie H, Wang Z, et al. Mooren’ ulcer in China: a study of clinical characteristics and treatment. Br J Ophthalmol. 2000; 84:1244–9.
9. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye treatment: effect on conjunctivla lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.
10. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.
11. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderated to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.
12. Hingorani M, Calder VL, Buckley RJ, Lightman S. The immunomodulatory effect of topical cyclosporin A in atopic keratoconjunctivitis. Invest Ophthalmol Vis Sci. 1999; 40:392–9.
13. Stern ME, Geo J, Siemasko KT, et al. Role of the lacrimal gland functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.
14. Pepose JS, Akata RF, Pflugfelder SC, et al. Mononuclear cell phenotypes and immunoglobulin gene rearrangements in larimal gland biopsies from patients with Sjogren's syndrome. Ophthalmology. 1990; 97:1599–605.
15. Borel JF, Baumann G, Chapman I, et al. In vivo pharmacological effects of ciclosporin and some analogues. Adv Pharmacol. 1996; 35:115–246.

16. Laibovitz RA, Solch S, Andriano K, et al. Pilot trial of cyclosrpoine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993; 12:315–23.
17. Power WJ, Mullaney P, Farrel M, et al. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjogren's syndrome. Cornea. 1993; 12:507–11.
18. Singh G, Lindstrom RL, Doughman DJ. Cyclosporin A on human corneal endothelium. Cornea. 1984; 3:272–7.

19. BenEzra D, Antebe I, Maftzir G. Differential effect of cyclosporin A on lymphocyte and keratocyte proliferation. Invest Ophthalmol Vis Sci. 1987; 28:42.
20. Garweg JG, Wegmann-Burns M, Goldblum D. Effects of daunorubicin, mitomycin C, azathiprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch Clin Exp Ophthalmol. 2006; 244:382–9.
21. Angelov O, Wiese A, Yuan Y, et al. Preclinical safety studies of cyclosporine ophthalmic emulsion. Adv Exp Med Biol. 1998; 438:991–5.

22. Tonoe O, Yamanaka O, Okada Y, et al. Collagen biosynthesis and cellular proliferation after filtering surgery in rabbits. Folia Ophthalmol Jpn. 1995; 46:242–5.
23. Berman B, Duncan MR. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycoaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989; 92:605–10.
24. Leonardi A, DeFranchis G, Fregona IA, et al. Effects of cyclosporine A on human conjunctival fibroblasts. Arch Ophthalmol. 2001; 119:1512–7.
25. Williamson MS, Miller EK, Plemons J, et al. Cyclosporine A upregulates interleukin-6 gene expression in human gingiva. J Periodontol. 1994; 65:895–903.
26. James JA, Irwin CR, Linden GJ. Gingival fibroblast response to cyclosporin A and transforming growth factor beta 1. J Periodontol Res. 1998; 33:40–8.
27. Adler R. Mechanism of photoreceptor death in retinal degeneration. From the cell biology of the 1990s to the ophthalmology of the 21st century. Arch Ophthalmol. 1996; 114:79–83.
28. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995; 267:1456–62.

29. Horigome A, Hirano T, Oka T, et al. Glucocorticoids and cyclosporine induce apoptosis in mitogen-acitvated human peripheral mononuclear cells. Immunopharmacology. 1997; 37:87–94.
30. Walter DH, Haendler J, Galle J, et al. Cylcosporin A inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation. 1998; 98:1153–7.
31. Healy E, Dempsey M, Lally C, Ryan MP. Apoptosis and necrosis. Kidney Int. 1998; 54:1955–66.
Go to : 
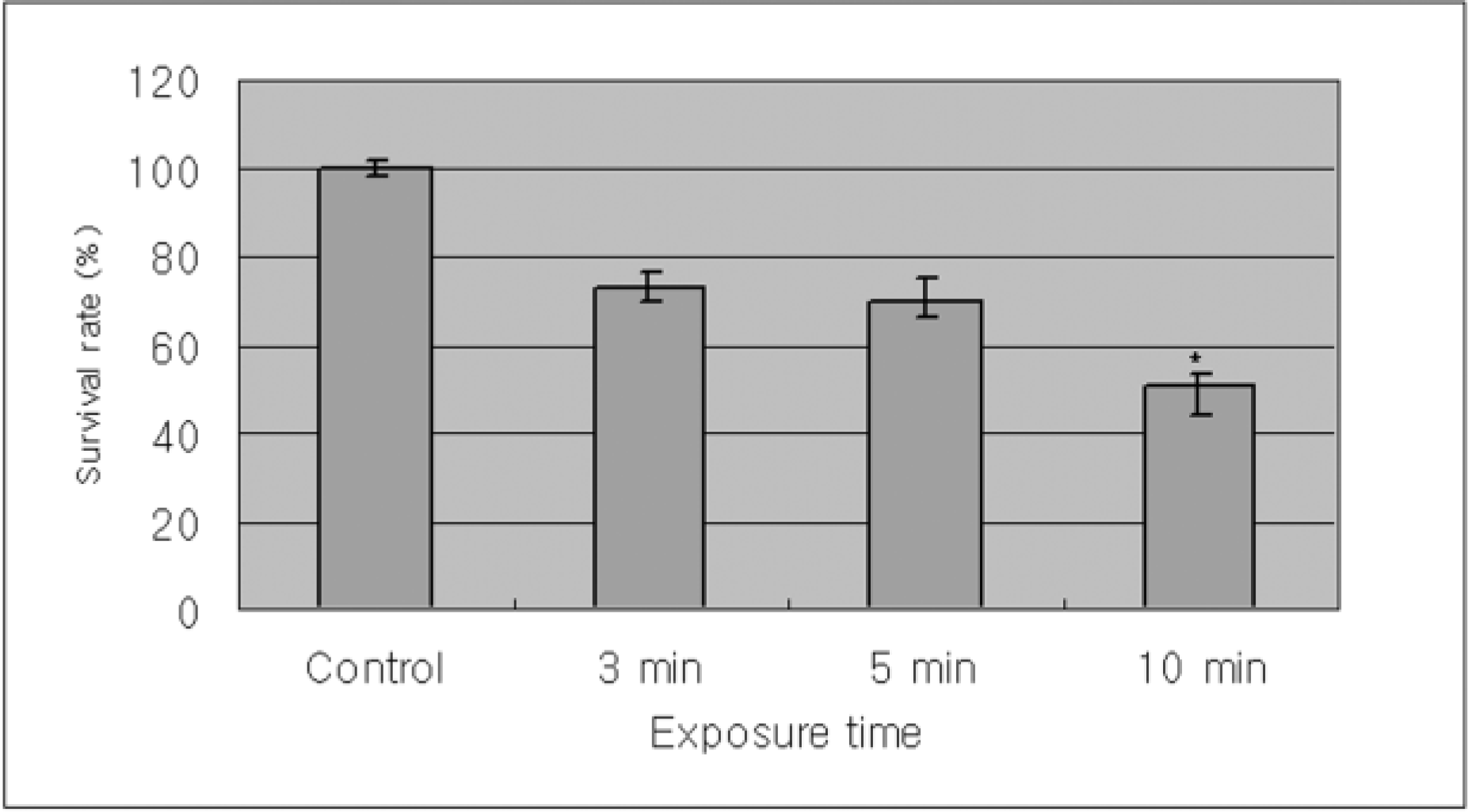 | Figure 1.Metabolic activities of cultured human corneal epithelial cells measured with MTT assays showed the time dependant response by the exposure time of cyclosporine. There is significant decrease in the proliferation of corneal epithelial cells comparing with control when exposed to cyclosporine A 0.05% for 10 minutes (p=0.04). |
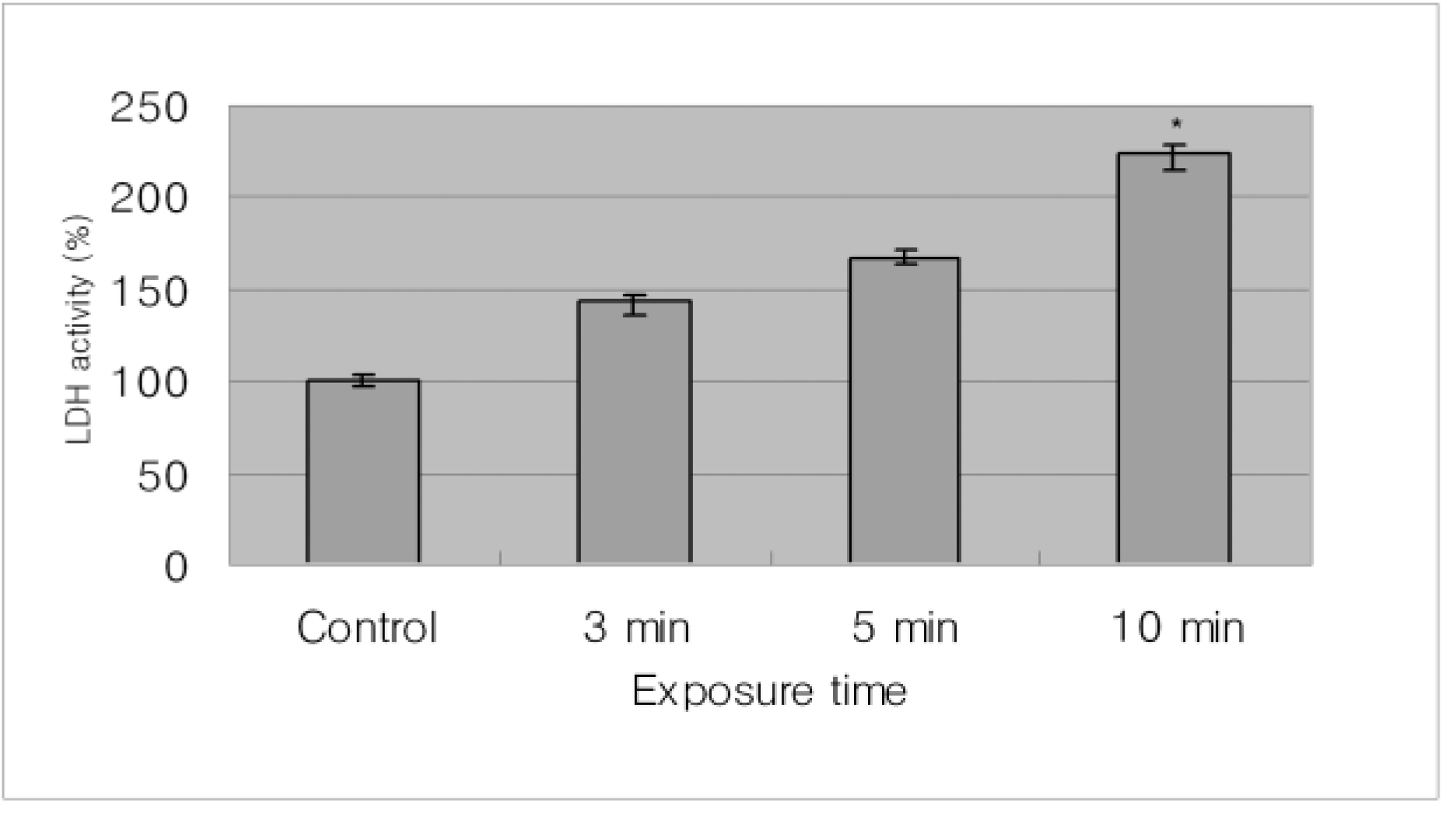 | Figure 2.With time, the LDH acitivity of cultured human corneal epithelial cells in cyclosporine A 0.05% showed time dependant response for cytotoxicity. There is significant increase in the cytotoxicity of corneal epithelail cells comparing with control when exposed to cyclosporine A 0.05% for 10 minutes (p=0.03). |
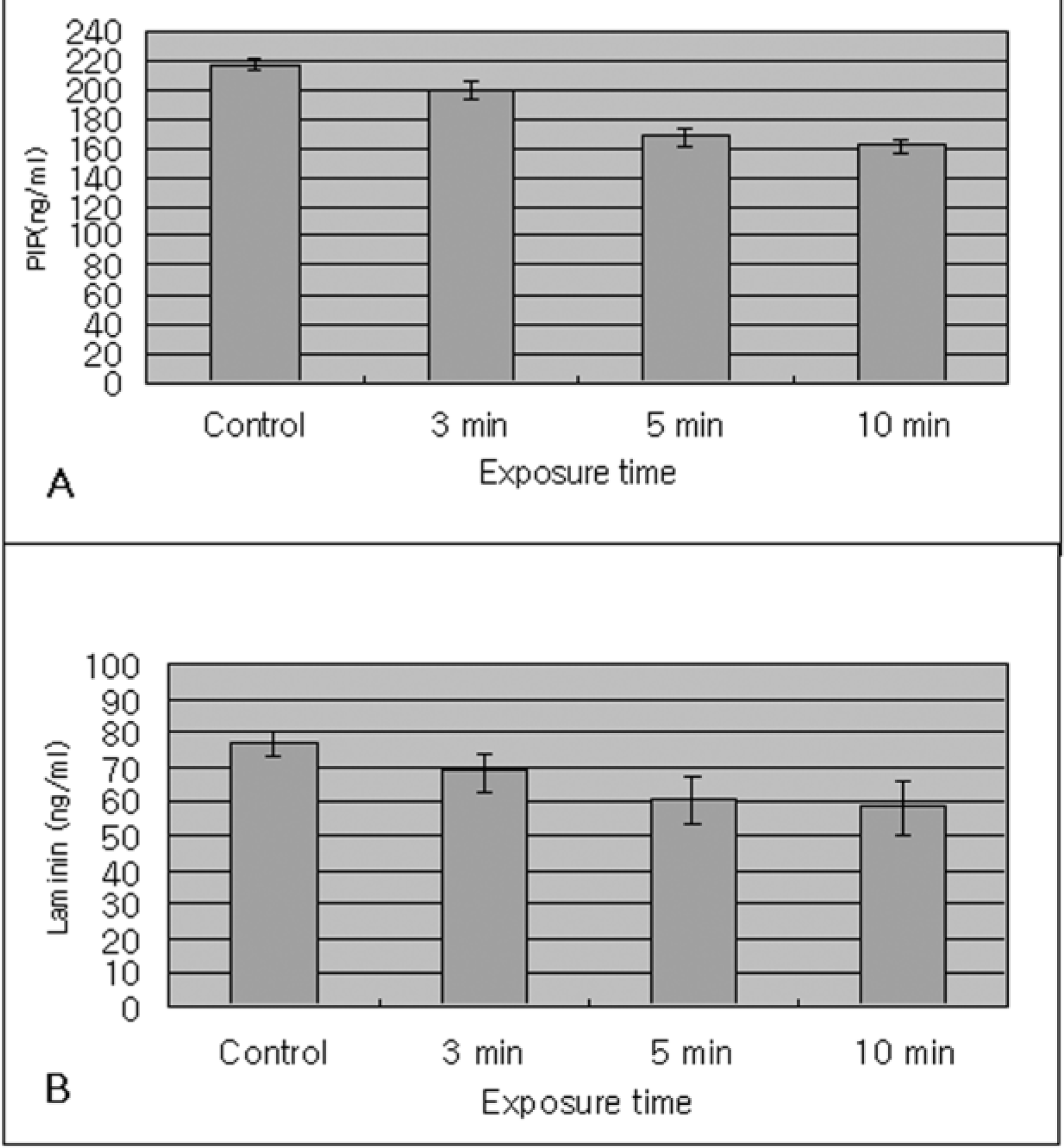 | Figure 3.In the immunoassay with extracellular matrix, the synthesis of type I collagen synthesis (PIP) (A) and the production of laminin (B) by cyclosporine A 0.05% showed a time dependent response in human corneal epithelium. |
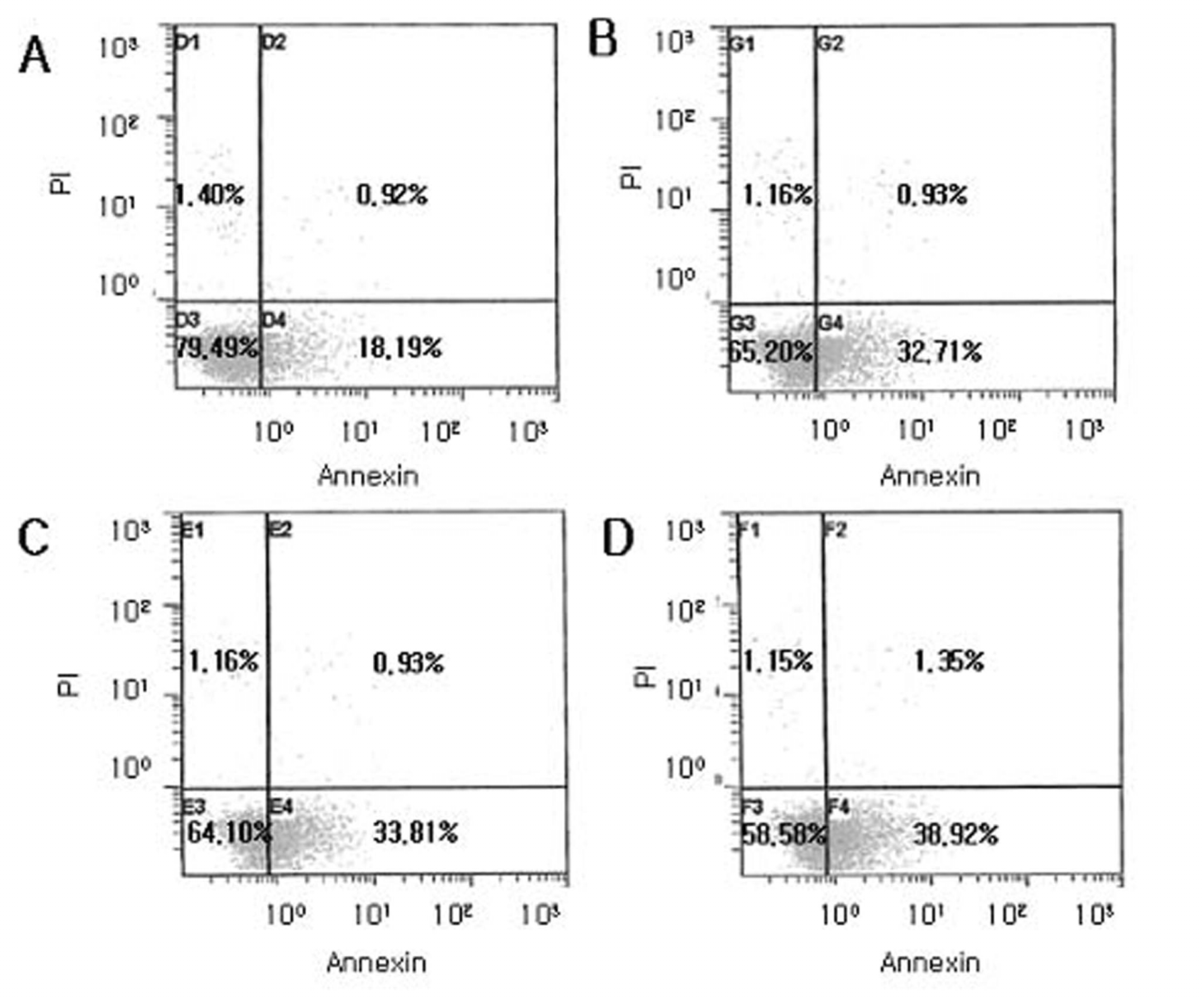 | Figure 4.Flow cytometric analysis of apoptotic cells using Annexin V FITC after exposure to cyclosporine. Human corneal epithelial cells were normal as control (A) or were treated with cyclosporine A 0.05% for 3 minutes (B), 5 minutes (C), and 10 minutes (D). Most normal epithelial cells were primarily Annexin V and PI negative (A). After treatment with cyclosporine A 0.05%, a significant number of cells are positive Annexin V and negative PI (B, C and D) indicating that the cells were in the stage of apoptosis. |
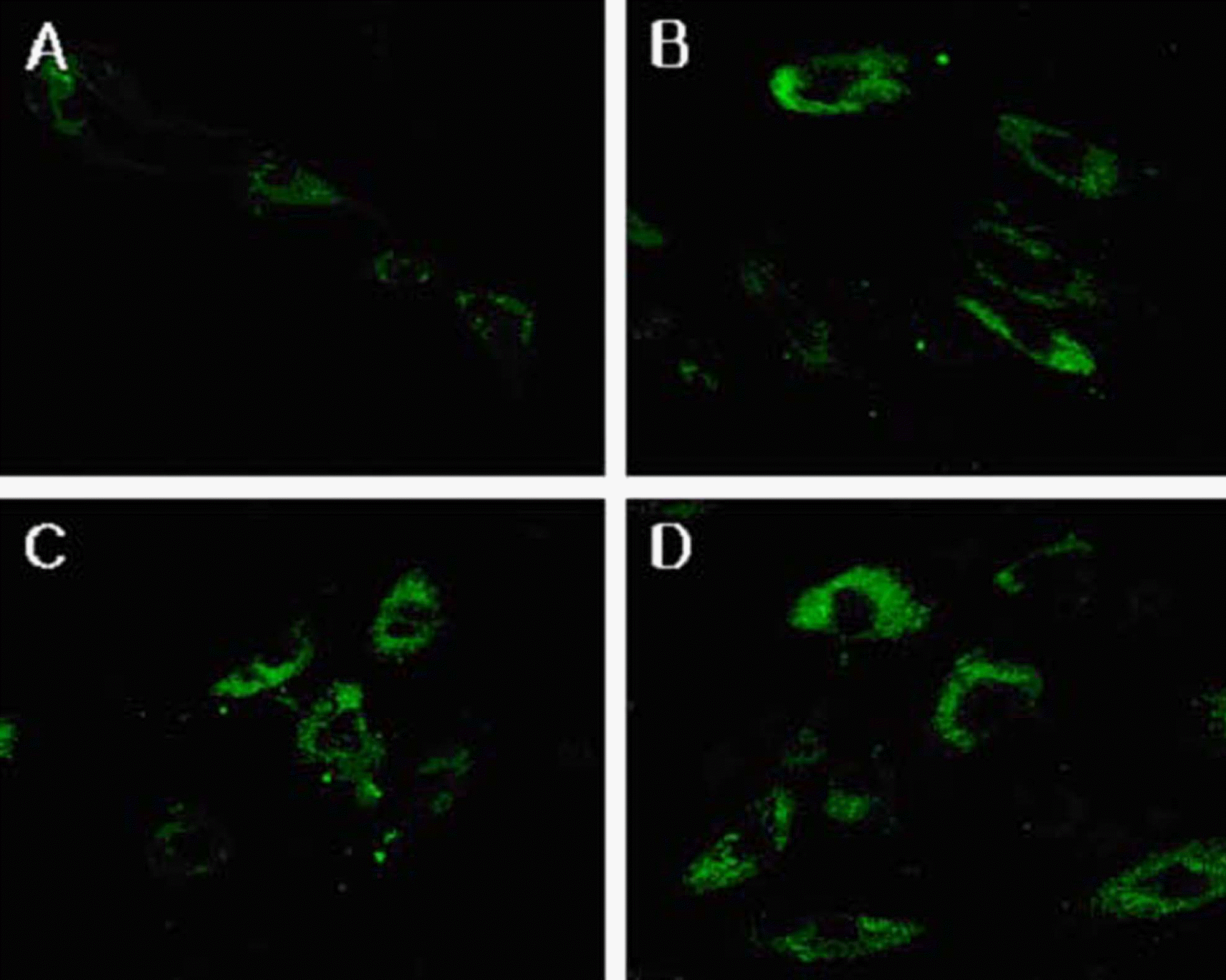 | Figure 5.Fluorescent micrograph of human corneal epithelial cells after exposure to cyclosporine A 0.05% (x 400): (A) control, (B) for 3 minutes, (C) for 5 minutes, (D) for 10 minutes. After treating with cyclosporine A 0.05%, apoptotic cells were visible, which represent fluorescence after binding DNA flowing into cytoplasm due to disruption of nuclear membrane. There were visible apoptotic bodies (white arrow) in extra cytoplasmic portion (B & C). |
 | Figure 6.Inverted phase contrast micrographs of human corneal epithelial cells after exposure to cyclosporine A 0.05% (*200): (A) control, (B) for 3 minutes, (C) for 5 minutes, (D) for 10 minutes. Many epithelial cells are visible in control culture media. There are less epithelial cells which are more detached from the culture media, in the case of exposure to cyclosporine A 0.05% compared with control. |
 | Figure 7.Transmission electron micrographs of human corneal epithelail cells after exposure to cyclosporine A 0.05% (bar length 2 um, A, B, C, & D : x3,000—4000); (A) control, (B) for 3 minutes, (C) for 5 minutes, (D) for 10 minutes. A normal corneal epithelial cell showed microvilli, homogenous cytoplasm and intact cell and nuclear membrane. The longer the exposure time of cyclosporine A 0.05%, the more decreased number of microvilli, well developed vacuole formation (white arrows), damaged mitochondria and rough endoplasmic reticulum (black arrows), and margination of chromatin in the nucleus (white arrowheads) the corneal epithelial cells revealed. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download