Abstract
Purpose
A filtering system (ABAK system, Thea, France) was developed and has been used to prevent the abuse of preservatives and to decrease the complications that may result from them. However, the bacteria filtering effect of the system has not been reported yet. In this study, we attempt to verify its efficacy.
Methods
Staphylococcus epidermidis was diluted to two different concentrations, 107 and 105 CFU (Colony-Forming-Unit)/ml. To determine the inward-filtering effect of the system (reverse direction), 0.5 ml of each bacterial concentration was aspirated through the ABAK system, and the solutions that filtered through were cultivated. The results were compared with the controls in which the same amounts of bacterial solutions were dropped from a dropper by squeezing the bottle. For the outward-effect (forward direction), 1ml of bacterial solution from each concentration was put into the bottle with a syringe. Solutions were re-collected by filtering them out through the ABAK system by squeezing the bottle and also by aspirating them from the bottle with a syringe. Both solutions were cultivated, and the results were compared. Each test was repeated 5 times.
Go to : 
References
1. Raynaud C, Laveran H, Rigal D, Bonicel P. Bacterial contamination of eyedrops in clinical use. J Fr Ophtalmol. 1997; 20:17–24.
2. Rahman MQ, Tejwani D, Wilson JA, et al. Microbial contamination of preservative free eye drops in multiple application containers. Br J Ophthalmol. 2006; 90:139–41.

3. Tasli H, Cosar G. Microbial contamination of eye drops. Cent Eur J Public Health. 2001; 9:162–4.
4. Aslund B, Olson OT, Sandell E. Studies on in-use microbial contamination of eye drops. Acta Pharm Suec. 1978; 15:389–94.
5. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal study. Ophthalmology. 1999; 106:556–63.
7. Schwab IR, Abbott RL. Toxic ulcerative keratopathy, An unrecognized problem. Ophthalmology. 1989; 96:1187–93.
8. Butt z, Kaufman D, McNab A, McKelvie P. Drug-induced ocular cicatricial pemphigoid: a series of clinico-pathological reports. Eye. 1998; 12:285–90.

9. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medications II. Outcomes of filtration surgery. Arch Ophthalmol. 1994; 112:1446–54.
10. Aaberg TM Jr, Flynn HW Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey. Ophthalmology. 1998; 105:1004–10.

11. Bannerman TL, Rhoden DL, McAllister SK, et al. The source of coagulase-negative staphylococci in the endophthalmitis vitrectomy study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997; 115:357–61.
12. Madaras-Kelly KJ, Ostergaard BE, Hovde LB, et al. Twenty- four hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996; 40:627–32.
13. Moreau JM, Girgis DO, Hume EBH, et al. Phospholipase A2 in rabbit tears: a host defense against Staphylococcus aureus. Invest Ophthalmol Vis Sci. 2001; 42:2347–54.
14. McCormick CC, Dajcs JJ, Reed JM, et al. The effectiveness of lysostaphin therapy for experimental coagulase-negative Staphylococcus endophthalmitis. Curr Eye Res. 2006; 31:225–30.
15. Baudin C, Pisella PJ, Glodschild M, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs. Human and animal studies. Ophthalmology. 1999; 3:556–63.
16. Debbasch C, Pisella PJ, De Saint Jean M, et al. Mitochondrial activity and glutathione injury in apoptosis induced by unpreserved and preserved (3-blockers on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001; 42:2525–33.
17. Young TL, Higginbotham EJ, Zou XL, Farber MD. Effects of topical glaucoma drugs on fistulized rabbit conjunctiva. Ophthalmology. 1990; 97:1423–7.
18. Broadway DC, Grierson I, Hitchings RA. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993; 77:590–6.

19. Ubels JL, McCartney MD, Lantz WK, et al. Effects of preservative-free artificial tear solutions on corneal epithelial structure and function. Arch Ophthalmol. 1995; 113:371–8.

20. Guenoun JM, Baudouin C, Rat P, et al. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci. 2005; 46:2444–50.

21. Ichijima H, Petroll WM, Jester JV, Cavanagh HD. Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride. Cornea. 1992; 11:221–5.

22. Rucker I, Kettrey R, Bach F, Zeleznick L. A safety test for contact lens wetting solutions. Ann Ophthalmol. 1972; 4:1000–6.
23. Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride in vitro. Clin Experiment Ophthalmology. 2004; 32:180–4.
Go to : 
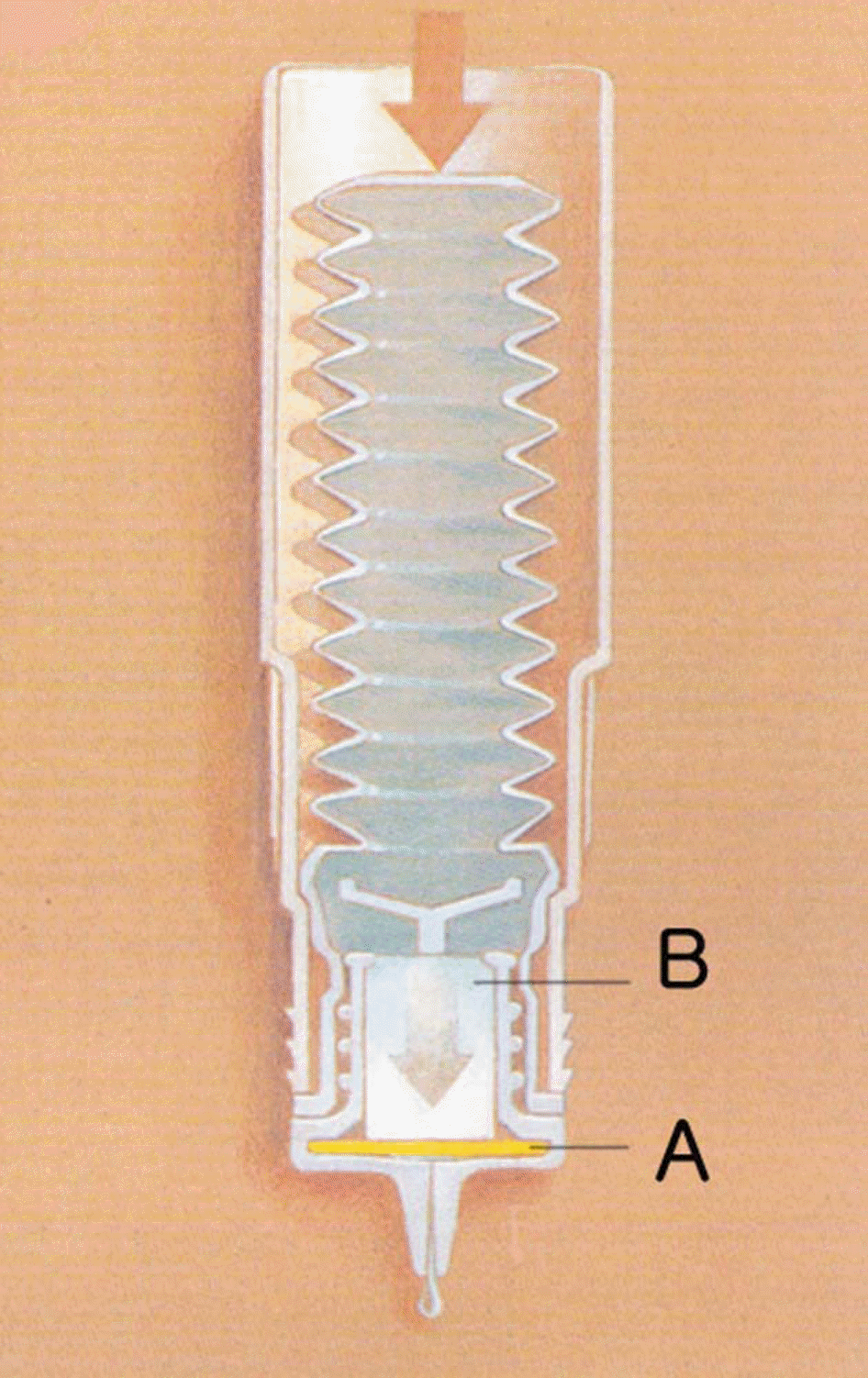 | Figure 1.The ABAKW system. (A) The filtering membrane, 0.2 gm micropore filter bacteria. (B) The nylon fiber cartridge to absorb the preservatives. |
Table 1.
Culture results with or without passing through the filter of NAABAK solution contaminated with S. epidermidis (reverse direction)
| No |
107 CFU/ml |
No |
105 CFU/ml |
||
|---|---|---|---|---|---|
| ∗Drop (colony) | † Bottle (colony) | ∗Drop (colony) | † Bottle (colony) | ||
| 1 | 564 | No gtrth | 6 | 9 | No gtrth |
| 2 | 850 | No gtrth | 7 | 3 | No gtrth |
| 3 | 215 | No gtrth | 8 | 6 | No gtrth |
| 4 | 511 | No gtrth | 9 | 7 | No gtrth |
| 5 | 564 | No gtrth | 10 | 5 | No gtrth |
| Mean | 540.8±225.6 | No gtrth | 6±2.2 | No gtrth | |
Table 2.
Culture results with or without passing through the filter of NAABAK solution contaminated with S. epidermidis (forward direction)
| No |
6×107 CFU/ml |
No |
6×105 CFU/ml |
||
|---|---|---|---|---|---|
| ∗Bottle (colony) | † Drop (colony) | ∗Bottle (colony) | † Drop (colony) | ||
| 11 | >103 | No gtrth | 16 | 275 | No gtrth |
| 12 | >103 | No gtrth | 17 | 197 | No gtrth |
| 13 | >103 | No gtrth | 18 | 188 | No gtrth |
| 14 | >103 | No gtrth | 19 | 205 | No gtrth |
| 15 | >103 | No gtrth | 20 | 253 | No gtrth |
| Mean | >103 | No gtrth | 223.6±38.2 | No gtrth | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download