Abstract
Purpose
To investigate the clinical manifestation and the structural optic disc changes according to the development of reproducible visual field defects in a group of preperimetric patients converting to early glaucoma.
Methods
Standard automated perimetry (Humphrey Field Analyzer) was performed every six months in 294 preperimetric patients. Each subject was classified as either converter or non-converter according to glaucomatous visual field changes, and the clinical manifestations were analyzed retrospectively. Sequential optic disc images were obtained using the TopSS scanning laser ophthalmoscope (TopSSTM) and optic disc parameters were measured to determine if any change had occurred.
Results
A total of 44 eyes from 44 subjects (14.9%) in 294 patients subsequently developed early glaucomatous field loss (converters). The progression rate of visual field defect is 0.43dB/year. In respect to age, sex, refractive error, and diabetes mellitus, no significant differences were observed. Elevated IOP, hypertension, and family history of glaucoma were detected more frequently in the converter group than in the non-converter group. Among TopSS parameters, cup-to-disc ratio, effective area, volume above, maximum slope, and neuroretinal rim area showed statistically significant change. Changes of the neuroretinal rim were prominent in superior and inferior sectors.
Conclusions
Among TopSS parameters, cup-to-disc ratio, effective area, volume above, and neuroretinal rim are useful in detecting the progression of glaucoma. Furthermore, neuroretinal rim changes in each sector may provide clinically relevant information in detecting and monitoring the progression of glaucoma.
Go to : 
References
1. Gupta N, Weinreb RN. New definition of glaucoma. Curr Opin Ophthalmol. 1997; 8:38–41.
2. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989; 107:453–64.

3. Kerrigan Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41:741–8.
4. Sommer A, Pollack I, Maumenee AE. Optic disc parameters and onset of glaucomatous field loss. Arch Ophthalmol. 1979; 97:1444–8.

5. Kruse FE, Burk RO, Voelcher HE, et al. Reproducibility of topographic measurements of the optic nerve head with laser tomographic scanning. Ophthalmology. 1993; 100:57–65.

6. Rohrschneider K, Burk RO, Kruse FE, Volker HE. Reproducibility of the optic nerve head topography with a new laser topographic scanning device. Ophthalmology. 1994; 101:1044–9.
7. Kamal DS, Garway-Heath DF, Hitchings RA, et al. Use of sequential Heidelberg retina tomograph images to identify changes at the optic disc in ocular hypertensive patients at risk of developing glaucoma. Br J Ophthalmol. 2000; 84:993–8.

8. O'corner DJ, Zeyen T, Caprioli J. Comparisons of methods to detect glaucomatous damage. Ophthalmology. 1993; 100:1498–503.
9. Weinreb RN, Lusky M, Bartsch D, et al. Effect of repetitive imaging on topographic measurements of the optic nerve head. Arch Ophthalmol. 1993; 111:636–8.

10. Nicolela MT, Drance SM. Various glaucomatous optic nerve head appearances: clinical correlations. Ophthalmology. 1996; 103:640–9.
11. Ahn CK, Ahn CS, Choi KR. Optic Disc Measurements with Topographic Scanning System. J Korean Ophthalmol Soc. 1997; 39:145–52.
12. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988; 29:1151–8.
13. Varma R, Tielsch JM, Quigley HA, et al. Race, age, gender, and refractive error related differences in the normal optic disc. Arch Ophthalmol. 1994; 112:1068–76.
14. Broad DC, Nicolela MT, Drance SM. Optic disk appearances in primary open angle glaucoma. Surv Ophthalmol. 1999; 43:223–43.
15. Lichter PR. Variability of expert observers in evaluating the optic disc. Trans Am Ophthalmol Soc. 1976; 74:532–72.
16. Kwon GR, Kee CW. Diagnostic criteria, its sensitivity, and specificity with confocal scanning laser ophthalmoscope TopSSTM. J Korean Ophthalomol Soc. 1999; 40:1036–42.
17. Ahn BS, Kee CW. Ability of a confocal scanning laser ophthalmoscope (TopSS) to detect early glaucomatous visual field defect. Br J Ophthalmol. 2000; 84:852–5.

18. Chung HS, Park MH, Kim HK, et al. Reproducibility of optic nerve head topographic measurements with the Heidelberg Retian Tomograph. J Korean Ophthalomol Soc. 1996; 37:136–42.
19. Uchida H, Brigatti L, Caprioli J. Detection of structural damage from glaucoma with confocal laser image analysis. Invest Ophthalmol Vis Sci. 1996; 37:2393–401.
20. Carpineto P, Ciancaglini M, Zuppardi E, et al. Reliability of nerve fiber layer thickness measurements using optical coherent tomography in normal and glaucomatous eyes. Ophthalmology. 2003; 110:190–5.
21. Hart WM Jr, Yablonski M, Kass MA, Becker B. Multivariate analysis of the risk of glaucomatous visual field loss. Arch Ophthalmol. 1979; 97:1455–8.

22. Wong TY, Klein BE, Klein R, et al. Refractive errors, intraocular pressure, and glaucoma in white population. Ophthalmology. 2003; 110:211–7.
23. Wilson MR, Hertzmark E, Walker AM, et al. A case control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987; 105:1066–71.
24. Tielsch JM, Katz J, Sommer A, et al. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994; 112:69–73.
25. Quigley HA, Brown ME, Morrison JD, Drance SM. The Size and shape of the optic disc in normal human eyes. Arch Ophthalmol. 1990; 108:51–7.

26. Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci. 1996; 37:1419–28.

27. O'Brien C, Schwartz B, Takamoto T, et al. Intraocular pressure and the rate of visual field loss in chronic open angle glaucoma. Am J Ophthalmol. 1991; 111:491–500.
28. Johnson CA, Adams AJ, Lewis RA. Evidence for a neural basis of age related visual field loss in normal observers. Invest Ophthalmol Vis Sci. 1989; 30:2056–64.
29. Tsai CS, Ritch R, Shin DH, et al. Age related decline of disc rim area in visually normal subjects. Ophthalmology. 1992; 99:29–35.
30. Funk J, Dieringer T, Grehn F. Correlation between neuroretinal rim area and age in normal subjents. Graefes Arch Clin Experiment Ophthalmol. 1989; 227:544–8.
31. Jonas JB, Budde WM, Panda Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999; 43:293–320.

32. Uhm KB, Lee DY, Lee JS, et al. Sensitivity and specificity of qualitative signs to detect glaucomatous optic nerve damage. J Korean Ophthalmol Soc. 1998; 39:152–62.
33. Airaksinen PJ, Drance SM. Neuroretinal rim area and retinal nerve fiber layer in glaucoma. Arch Ophthalmol. 1985; 103:203–4.

34. Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993; 100:63–8.

35. Kim JH, Baek CE, Ahn YK, et al. Pattern of glaucomatous optic disc damage in primary open angle glaucoma. J Korean Ophthalomol Soc. 1997; 38:1037–43.
Go to : 
 | Figure 1.Diagram of neuroretinal rim area in 12 sectors in the left eye. Each sectors counted in a clockwise manner in the left eyes and in a counterclockwise in the right eyes. (12 o'clock=superior, 3 o'clock=temporal). |
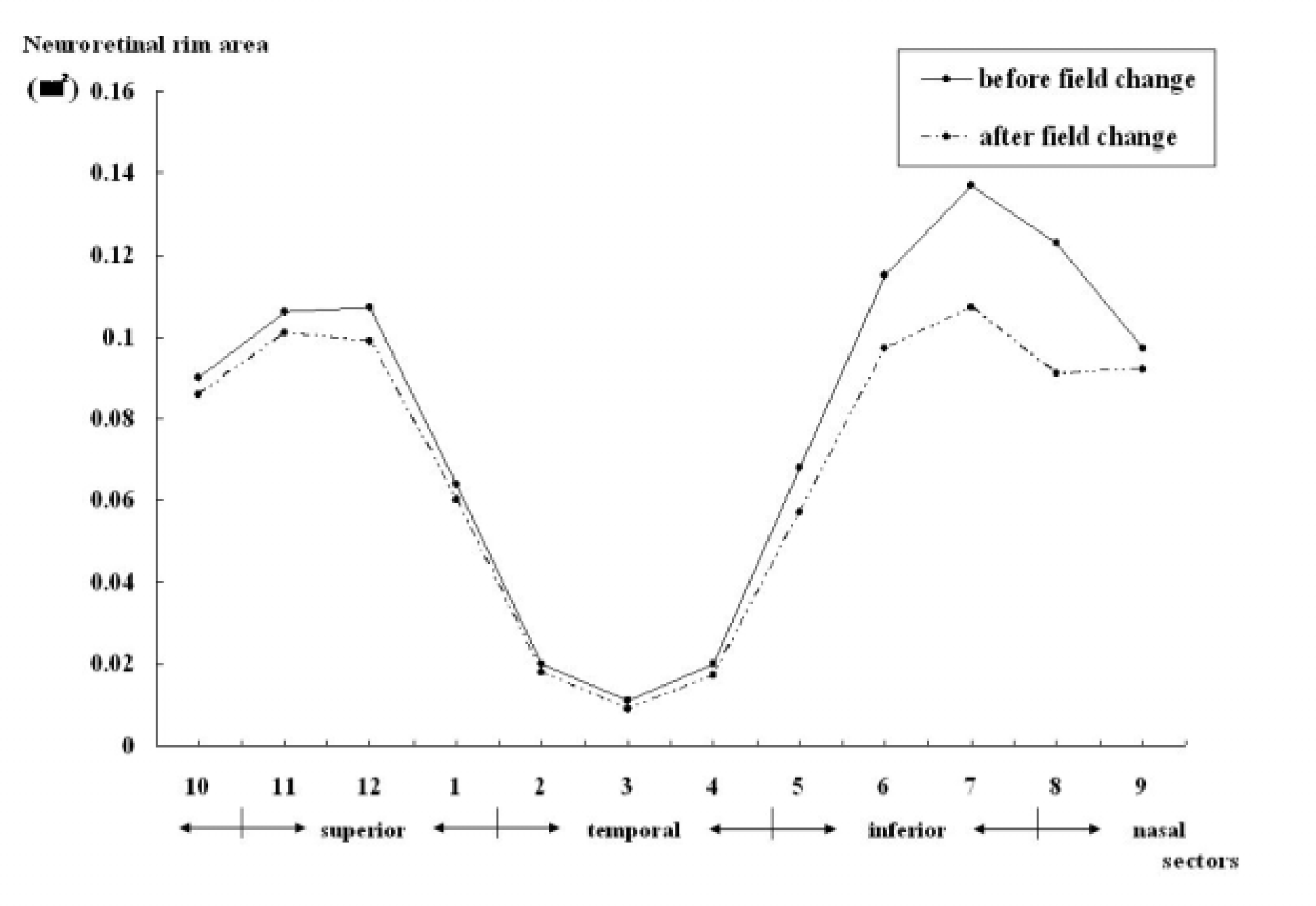 | Figure 2.Comparisons of neuroretinal rim area by sector within converter group Each sectors counted in a clockwise manner in the left eyes and in a counterclockwise in the right eyes. |
Table 1.
Baseline demographics and visual field indices in converter and non-converter group
| Converter | Non-converter | p-value∗ | |
|---|---|---|---|
| No. of eyes (%) | 44 (14.9) | 250 (85.1) | − |
| Age (years) | 54.1±13.5 | 51.3±9.8 | NS |
| IOP (mm Hg) | 19.5±3.7 | 14.2±6.2 | p<0.05 |
| RE (diopter) | −0.75±3.53 | −1.23±2.54 | NS |
| MD (dB) | −1.49±1.75 | −1.62±2.21 | NS |
| PSD (dB) | 1.58±2.43 | 1.71±2.87 | NS |
| Follow-up period (months) | 53.8±17.1 | 47.8±19.9 | NS |
Table 2.
Sex distribution and risk factors in converter and non-converter group
| Converter | Non-converter | p-value | |
|---|---|---|---|
| Gender | |||
| Male (%) | 26 (59) | 147 (59) | NS∗ |
| Female (%) | 18 (41) | 103 (41) | |
| HTN (%) | 28 (64) | 32 (3) | p<0.05† |
| DM (%) | 11 (25) | 78 (31) | NS† |
| Family history of Glaucoma (%) | 10 (23) | 1 (0.4) | p<0.05† |
| No. of eyes (%) | 44 (100) | 250 (100) |
Table 3.
Baseline topographic parameter between converter and non-converter group
| unit | Converter | Non-converter | p-value∗ | |
|---|---|---|---|---|
| Mean contour depth | mm | −0.11±0.12 | −0.10±0.14 | NS |
| Effective area | mm2 | 1.40±0.56 | 1.34±0.34 | p<0.05 |
| Average depth | mm | −0.28±0.07 | −0.29±0.10 | NS |
| Volume above | mm3 | 0.16±0.09 | 0.18±0.03 | p<0.05 |
| Volume below | mm3 | −0.40±0.21 | −0.44±0.31 | NS |
| 1/2 depth area | mm2 | 0.64±0.38 | 0.63±0.24 | NS |
| 1/2 depth volume | mm3 | −0.09±0.07 | −0.09±0.05 | NS |
| Maximum depth | mm | −0.64±0.21 | −0.83±0.10 | NS |
| Maximum slope | degree | 77.66±2.93 | 78.97±4.47 | NS |
| Average slope | degree | 35.22±5.01 | 36.80±6.02 | NS |
| Contour variation | mm | 0.34±0.12 | 0.36±0.11 | NS |
| Cup shape | − | −0.05±0.03 | −0.05±0.05 | NS |
| C/D ratio | − | 0.62±0.17 | 0.59±0.15 | NS |
| Horizontal C/D ratio | − | 0.61±0.19 | 0.60±0.14 | NS |
| Vertical C/D ratio | − | 0.62±0.18 | 0.59±0.21 | NS |
| NRRA | mm2 | 0.97±0.38 | 0.96±0.20 | NS |
Table 4.
Change of visual field indices within converter group
| Baseline | At the time of conversion | p-value∗ | |
|---|---|---|---|
| MD (dB) | −1.49± 1.75 | −3.36± 1.62 | p<0.05 |
| PSD (dB) | 1.58±2.43 | 2.43± 1.33 | p<0.05 |
Table 5.
Comparisons of topographic parameters within converter group according to development of glaucomatous visual field defect
| unit | Baseline | At the time of conversion | p-value∗ | |
|---|---|---|---|---|
| Mean contour depth | mm | −0.10±0.12 | −0.11±0.14 | NS |
| Effective area | mm2 | 1.40±0.56 | 1.46±0.63 | p<0.05 |
| Average depth | mm | −0.28±0.07 | −0.27±0.10 | NS |
| Volume above | mm3 | 0.16±0.09 | 0.13±0.07 | p<0.05 |
| Volume below | mm3 | −0.40±0.21 | −0.42±0.27 | NS |
| 1/2 depth area | mm2 | 0.64±0.38 | 0.62±0.36 | NS |
| 1/2 depth volume | mm3 | −0.09±0.07 | −0.09±0.08 | NS |
| Maximum depth | mm | −0.64±0.20 | −0.61±0.20 | NS |
| Maximum slope | degree | 77.66±2.93 | 76.44±3.75 | p<0.05 |
| Average slope | degree | 35.22±5.01 | 34.63±6.20 | NS |
| Contour variation | mm | 0.34±0.12 | 0.36±0.11 | NS |
| Cup shape | − | −0.05±0.03 | −0.05±0.05 | NS |
| C/D ratio | − | 0.62±0.17 | 0.74±0.15 | p<0.05 |
| Horizontal C/D ratio | − | 0.61±0.19 | 0.82±0.14 | p<0.05 |
| Vertical C/D ratio | − | 0.62±0.18 | 0.72±0.11 | p<0.05 |
| NRRA | mm2 | 0.97±0.38 | 0.81±0.32 | p<0.05 |
Table 6.
Comparisons of neuroretinal rim area by sector within converter group
| Sector(mm2) | Disc sectors† | Baseline | At the time of conversion | p-value∗ |
|---|---|---|---|---|
| Superior | 12 | 0.11±0.05 | 0.10±0.04 | p<0.05 |
| Superotemporal | 1+2 | 0.04±0.03 | 0.04±0.04 | NS |
| Temporal | 3 | 0.01±0.02 | 0.01±0.02 | NS |
| Inferotemporal | 4+5 | 0.04±0.03 | 0.03±0.03 | p<0.05 |
| Inferior | 6 | 0.12±0.04 | 0.10±0.04 | p<0.05 |
| Inferonasal | 7+8 | 0.13±0.05 | 0.10±0.04 | p<0.05 |
| Nasal | 9 | 0.10±0.06 | 0.09±0.04 | NS |
| Superonasal | 10+11 | 0.10±0.05 | 0.09±0.04 | NS |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download