Abstract
Purpose
To investigate the effect of intravitreal injection of triamcinolone acetonide (IVTA) for the treatment of macular edema in central retinal vein occlusion (CRVO).
Methods
Seventy patients with CRVO and persistent macular edema were included in this retrospective study. Thirty-six eyes of 36 patients received an intravitreal injection of 4 mg (0.1 cc) triamcinolone and 34 eyes of 34 control patients didnot receive an injection. Any differences in visual acuity and foveal thickness were measured and compared between the two groups. The correlation between the change in foveal thickness and visual acuity was also evaluated.
Results
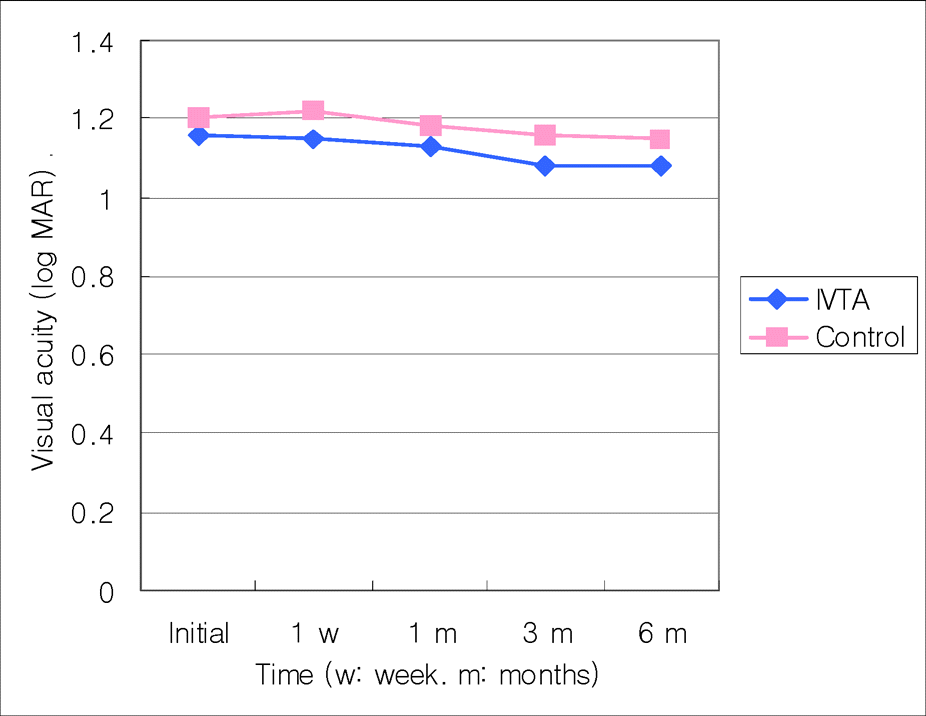
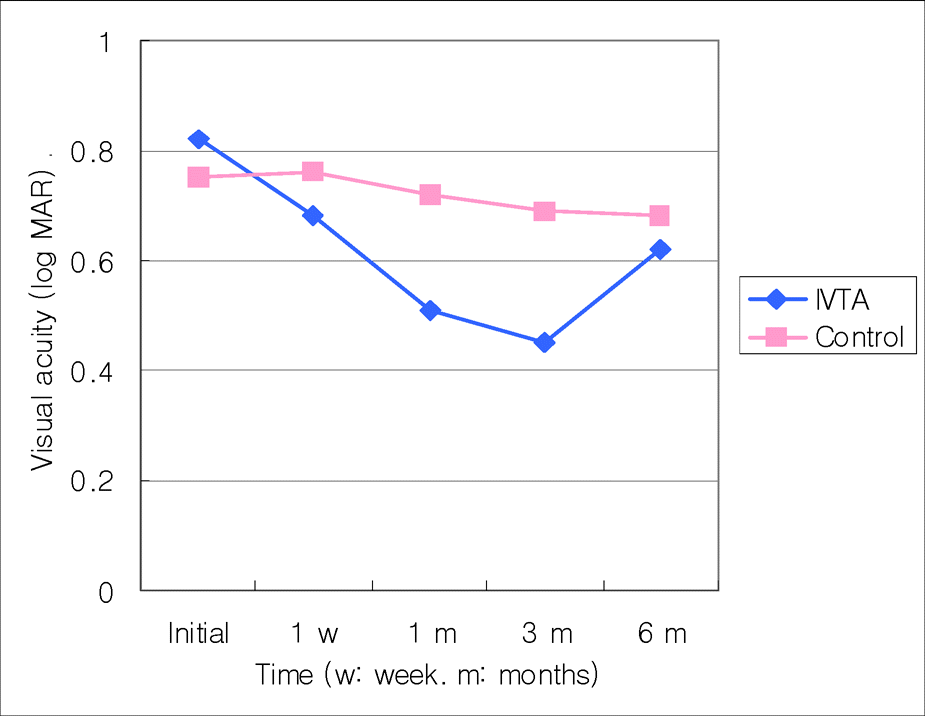
In ischemic CRVO group, the mean difference of visual acuity between the injection and the control groups was not statistically significant (p<0.05) at the initial measurement and at one week, one, three, and six months after the injection. However, in the non-ischemic CRVO group it was not clinically significant (p<0.05) at the initial time, one week or six months after the injection. But it was clinically significant at one months and three months after receiving the injection (p=0.004 and 0.001). The mean difference in foveal thickness was clinically significant (p<0.001). The visual acuity and foveal thickness were correlated in non-ischemic group but not in the ischemic group.
Go to : 
References
1. Bashshur ZF, Ma'luf RN, Allam S, et al. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1137–40.
2. Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981; 79:371–422.
3. Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997; 46:1473–80.

4. Vinores SA, Youssri AO, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
5. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105:412–6.
6. Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998; 341:309–15.

7. Nauck M, Roth M, Tamm M, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol. 1997; 16:398–406.

8. Jonas JB, Jreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61.

9. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109:920–7.

10. Young S, Larkin G, Braniey M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001; 29:2–6.

11. Benhamou N, Massin P, Haouchine B, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol. 2003; 135:246–9.

12. Jung HJ, Hyun JH, Kim YI, Yun IH. Normal macular thickness measured macular mapping of OCT3. J Korean Ophthalmol Soc. 2004; 45:962–8.
13. The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995; 102:1425–33.
14. Elman MJ, Raden RZ, Carrigan A. Intravitreal injection of tissue pasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2001; 99:219–21.
15. Opremcak EM, Bruce RA, Lomeo M, et al. Radial optic neurotomy for central retinal vein occlusion. Retina. 2001; 21:408–15.

16. Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology. 2001; 108:2249–57.

17. McAllister IL, Douglas JP, Constable IJ, Yu DY. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion. Am J Ophthalmol. 1998; 126:219–29.
18. Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002; 120:1217–9.

19. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.

20. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Experiment Ophthalmol. 2002; 240:782–3.

21. Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1131–6.
Go to : 
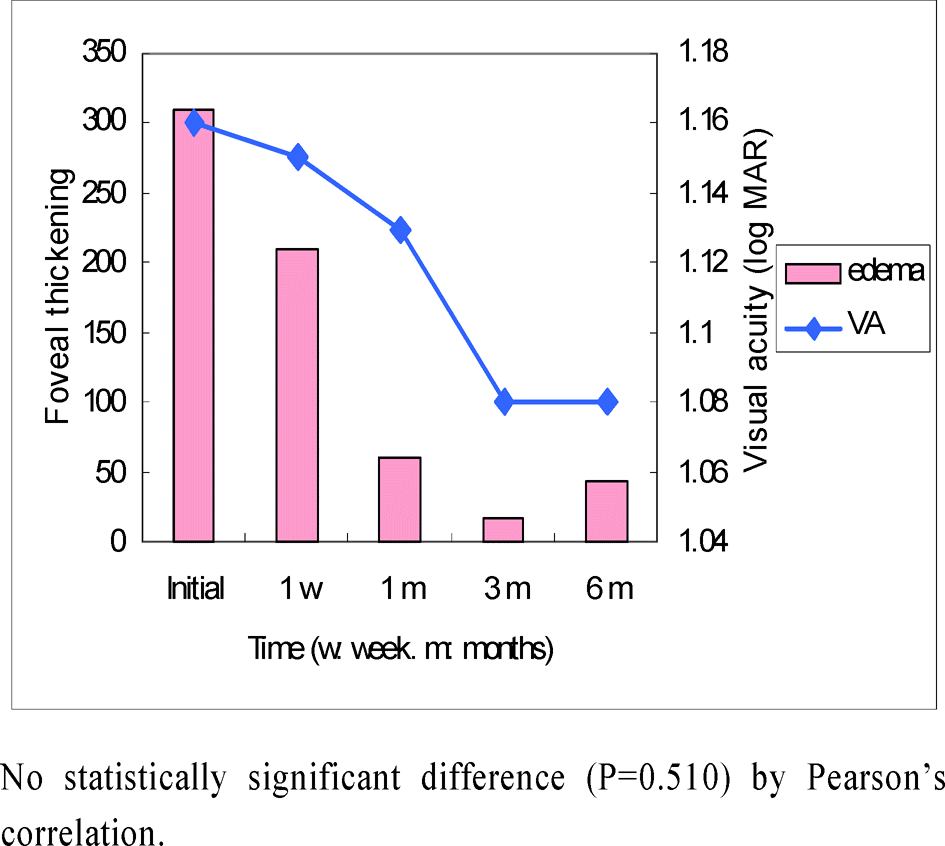 | Figure 3.Correlation of macular edema and visual acuity in IVTA group of ischemic central retinal vein occlusion patients. |
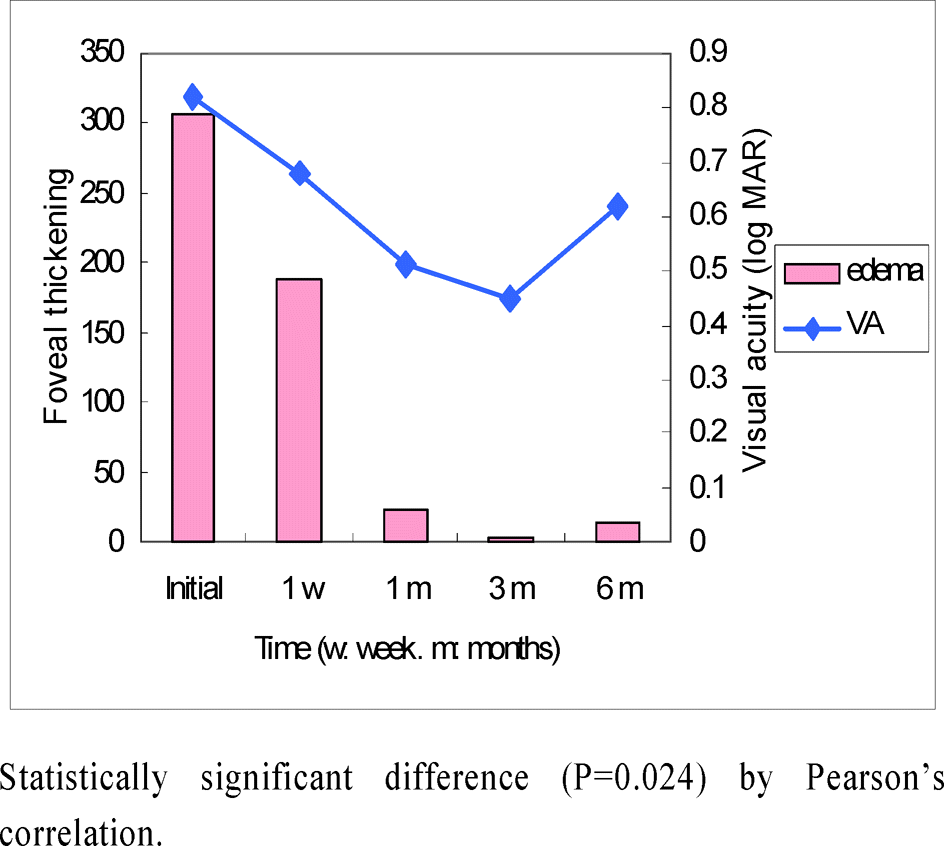 | Figure 4.Correlation of macular edema and visual acuity in IVTA group of non-ischemic central retinal vein occlusion patients. |
Table 1.
Baseline characteristics
|
IVTA group |
Control group |
|||
|---|---|---|---|---|
| Ischemic | Non-ischemic | Ischemic | Non-ischemic | |
| No. of eyes | 20 | 16 | 18 | 16 |
| Male:Female | 8:12 | 4:12 | 6:12 | 4:12 |
| Mean age (age) | 53.0±13.5 | 56.3±24.5 | 59.2±12.1 | 51.8±10.5 |
| Mean follow-up time (months) | 6.4±1.9 | 7.4±2.6 | 5.9±0.7 | 8.4±3.2 |
| Baseline visual acuity (logMAR) | 1.16 | 0.82 | 1.20 | 0.75 |
| Hypertention∗ | 7 | 6 | 4 | 7 |
| Coronary artery | 1 | 2 | 3 | 1 |
| disease∗ | ||||
| Smoker∗ | 4 | 3 | 5 | 3 |
Table 2.
Improvement of final visual acuity
|
IVTA group (%) |
Control group (%) |
|||
|---|---|---|---|---|
| Ischemic | Non-ischemic | Ischemic | Non-ischemic | |
| Improved∗ | 5/20 (25) | 13/16 (81)† | 4/18 (22) | 12/16 (75)† |
| Unchanged | 6/20 (30) | 2/16 (13) | 5/18 (28) | 3/16 (19) |
| Worsen | 9/20 (45) | 1/16 (6) | 9/18 (50) | 1/16 (6) |
Table 3.
Changes of foveal thickness (gm)
|
Foveal thickening† : mean±SD (μm) |
||
|---|---|---|
| Ischemic (n=20)∗ | Non-ischemic (n=16)∗ | |
| Initial | 310±43 | 307±51 |
| 1 week | 209±52 | 187±69 |
| 1 month | 60±92 | 23±88 |
| 3 month | 18±59 | 4±47 |
| 6 month | 43±87 | 15±78 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download