Abstract
Purpose
We investigated the therapeutic effects of monoclonal anti-TNF antibody (infliximab) on experimental uveitis.
Methods
Twenty New Zealand White rabbits were immunized with Mycobacterium tuberculosis H37Ra antigen and then challenged with intravitreal injection of tuberculin antigen to introduce a uveitis. Then infliximab was injected into rabbit eyes at an intravenous concentration of 5 mg/kg and intravitreal concentrations of 1 mg/0.1mL and 100 microg/0.1mL. As a control, the vehicle was injected intravenously or intravitreally. To evaluate the therapeutic effects, inflammation was assessed by slit lamp biomicroscopy and scored according to the severity of inflammation. The animals were also evaluated by electroretinography and histopathology.
Results
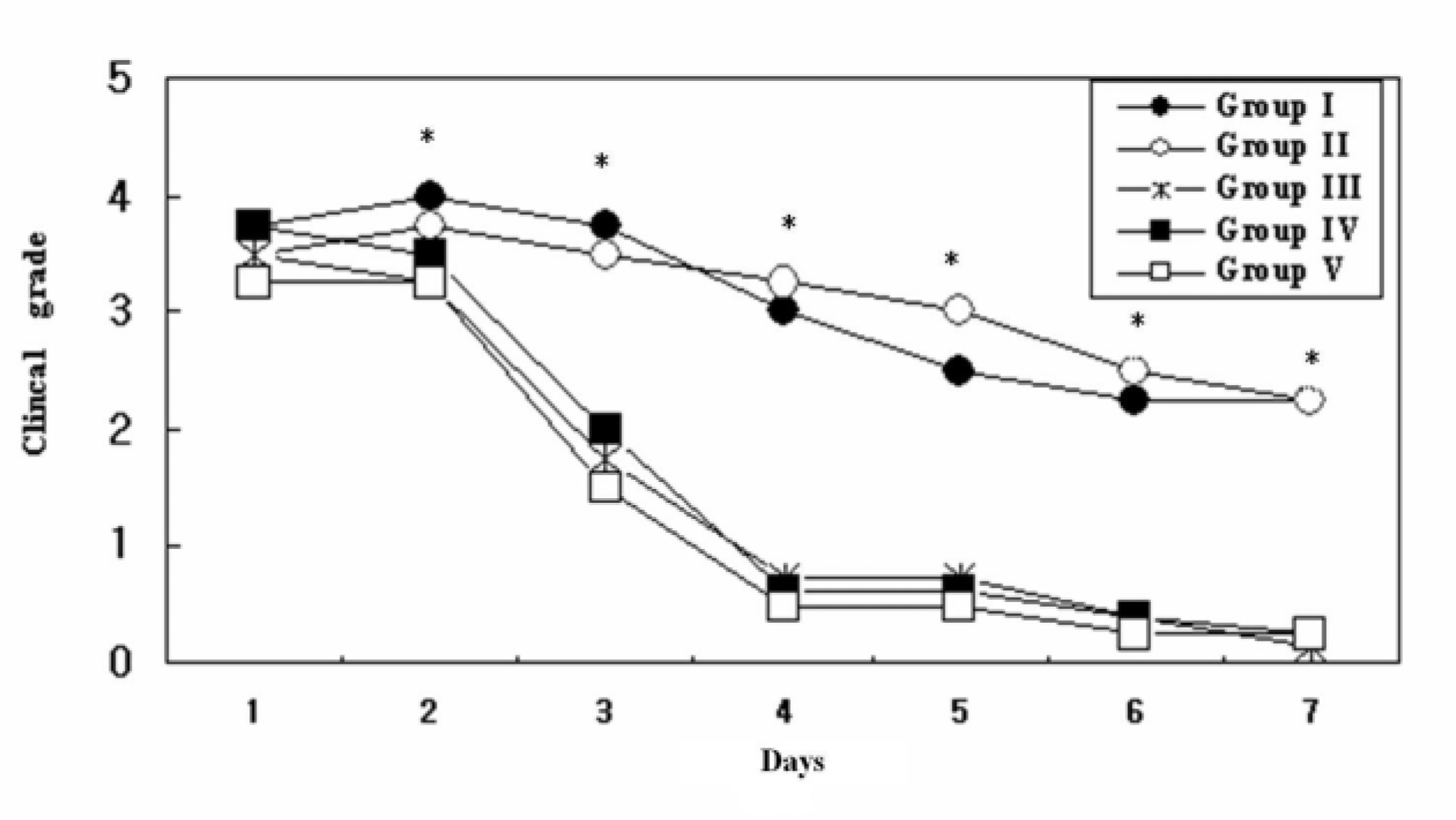
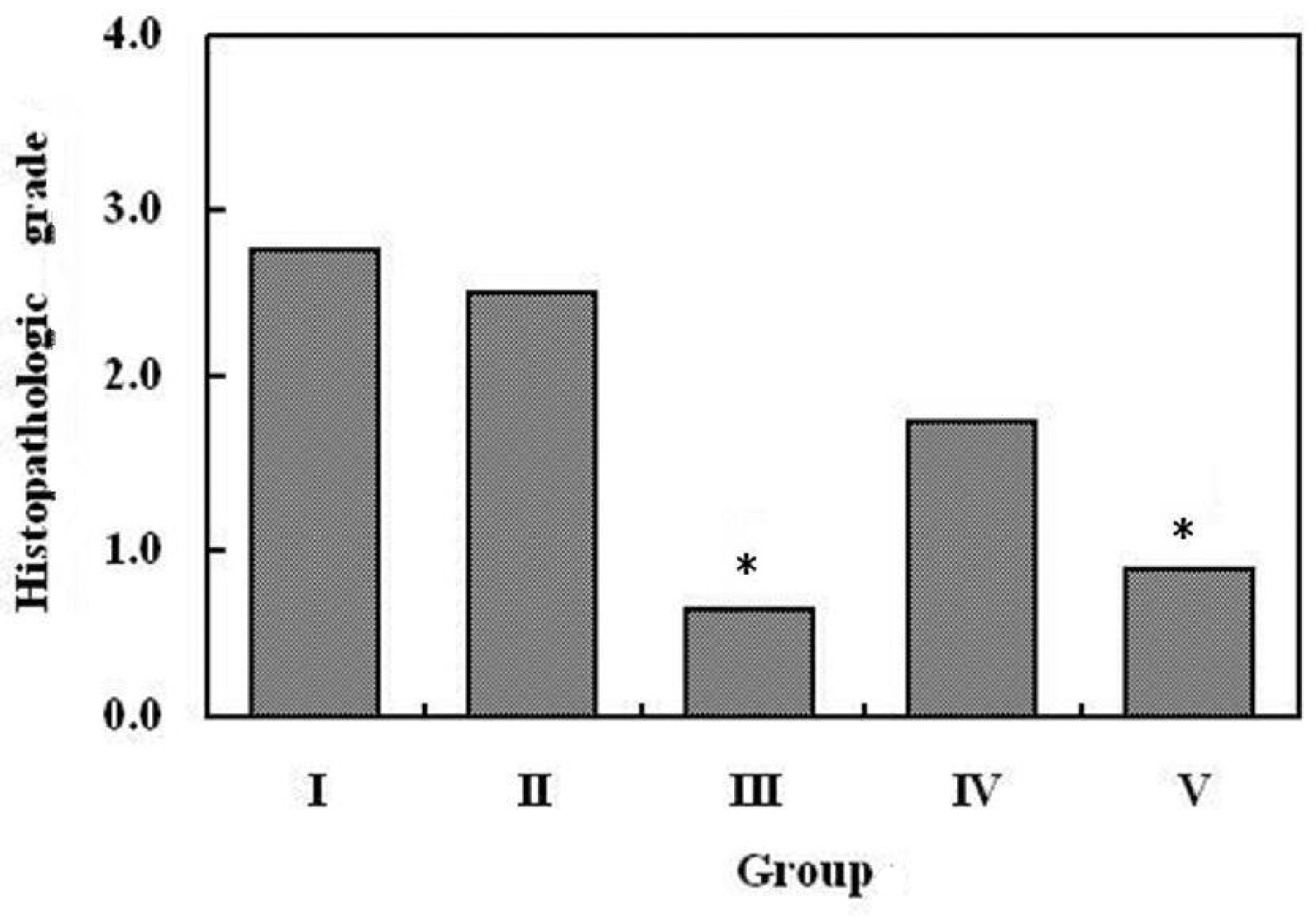
Regardless of the administration route, inflammatory activities of anterior chamber and engorgement of vascular structures were reduced in the infliximab treated group compared to control. Different administration routes and different concentrations of infliximab did not affect the therapeutic outcome of the clinical scoring. Intravenous (5 mg/kg) and intravitreal diluted (100 microg/0.1mL) infliximab injection groups showed significant improvement in electroretinographic findings and significant reduction of inflammatory cells with preservation of retinal tissue architecture on histopathologic examination. However, focal loss of the photoreceptor outer segment is observed in intravitreal undiluted (1 mg/0.1 mL) infliximab injected eyes. Conclusions: Infliximab may be a useful treatment modality to suppress ocular inflammation in experimental uveitis models in rabbits.
Go to : 
References
1. Nussenblatt RB, Whitecup SM, Palestine AG. Uveitis, Fundamentals and Clinical practice. 2nd ed.St. Louis: Mosby;1996. p. 18–152.
2. Becker MD, Adamus G, Davey MP, Rosenbaum JT. The role of T cells in autoimmune uveitis. Ocul Immunol Inflamm. 2000; 8:93–100.

3. Boyd SR, Young S, Lightman S. Immunopathology of the noninfectious posterior and intermediate uveitides. Surv Ophthalmol. 2001; 46:209–33.

4. Illei GG, Lipsky PE. Novel, non-antigenic-specific therapeutic approaches to autoimmune/inflammatory disease. Curr Opin Immunol. 2000; 12:712–8.
5. Brandt J, Haibel H, Cornely D, et al. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum. 2000; 43:1346–52.
6. Baert FJ, D'Haens GR, Peeters M, et al. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999; 116:22–8.
7. Elliot MJ, Maini RN, Feldmann M, et al. Randomized double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994; 344:1105–10.
8. Jaffe GJ, Yang CS, Wang XC, et al. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology. 1998; 105:46–56.

9. Aydin E, Kazi AA, Peyman GA, Esfahani MR. Intravitreal toxicity of moxifloxacin. Retina. 2006; 26:187–90.

10. Ishikawa T, Hokama H, Katagiri Y, et al. Effects of intravitreal injection of tacrolimus (FK506) in experimental uveitis. Curr Eye Res. 2005; 30:93–101.

11. Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004; 102:395–419.

13. Bamforth SD, Lightman S, Greenwood J. The effect of TNF-a and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol. 1996; 32:624–32.
14. Koizumi K, Poulaki V, Doehmen S, et al. Contribution of TNF-ato leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003; 44:2184–91.
15. Sartani G, Silver PB, Rizzo LV, et al. Anti-tumor necrosis factor alpha theraphy suppresses the induction of experimental autoimmune uveoretinitis in mice inhibiting antigen priming. Invest Ophthalmol Vis Sci. 1996; 37:2211–8.
16. Feldman M, Taylor P, Paleolog E, et al. Anti-TNFa therapy is useful in rheumatoid arthritis and Crohn's disease: Analysis of the mechanism of action predicts utility in other disease. Transplantation proceeding. 1998; 30:4126–7.
17. Sfikakis PP, Theodossiadis PG, Katsiari CG, et al. Effect of infliximab on sight-threatening panuveitis in Behcet's disease. Lancet. 2001; 358:295–6.

18. Sfikakis PP. Behcet's disease: a new target for anti-tumor necrosis factor treatment. Ann Rheum Dis. 2002; 61:51–3.
19. Sfikakis PP, Kaklamanis PH, Elezologlou A, et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behget's disease. Ann Intern Med. 2004; 140:404–6.
20. Triolo G, Vadala M, Accardo-Palumbo A, et al. Anti-tumour necrosis factor monoclonal antibody treatment for ocular Behcet's disease. Ann Rheum Dis. 2002; 61:560–1.

21. Hwang JH, Shin SY. Anti-tumor necrosis factor monoclonal antibody treatment for Chronic Behcet's disease: 3 cases. J Korean Ophthalmol Soc. 2005; 46:1405–10.
22. Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005; 123:903–12.
23. Joseph A, Raj D, Dua HS, et al. Infliximab in the treatment of refractory posterior uveitis. Ophthalmology. 200l; 110:1449–53.

24. Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005; 129:1117–20.

25. Strong BY, Erny BC, Herzenberg H, Razzeca KJ. Retrobulbar optic neuritis associated with infliximab in a patient with Crohn disease. Ann Intern Med. 2004; 140:34.

26. Foroozan R, Buono LM, Sergott RC, Savino PJ. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol. 2002; 120:985–7.
27. Ohno S, Nakamura S, Hori S, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet's disease with refractory uveoretinitis. J Rheumatol. 2004; 31:1362–8.
Go to : 
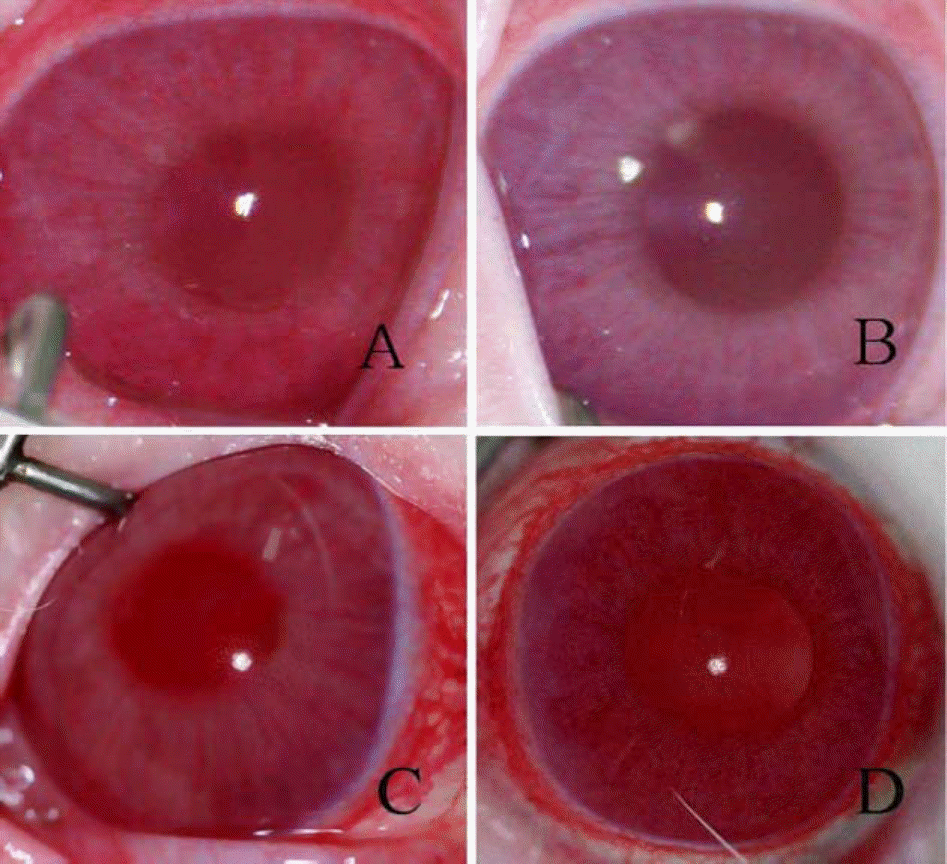 | Figure 1.In the treated eye, dilated iris blood vessel and hazy anterior chamber (A) improved after intravenous infliximab treatment (B) In contrast, the untreated eye showed marked conjunctival injection and inflammation of the anterior chamber (C) and unchanged after intravenous BSS treatment (D). |
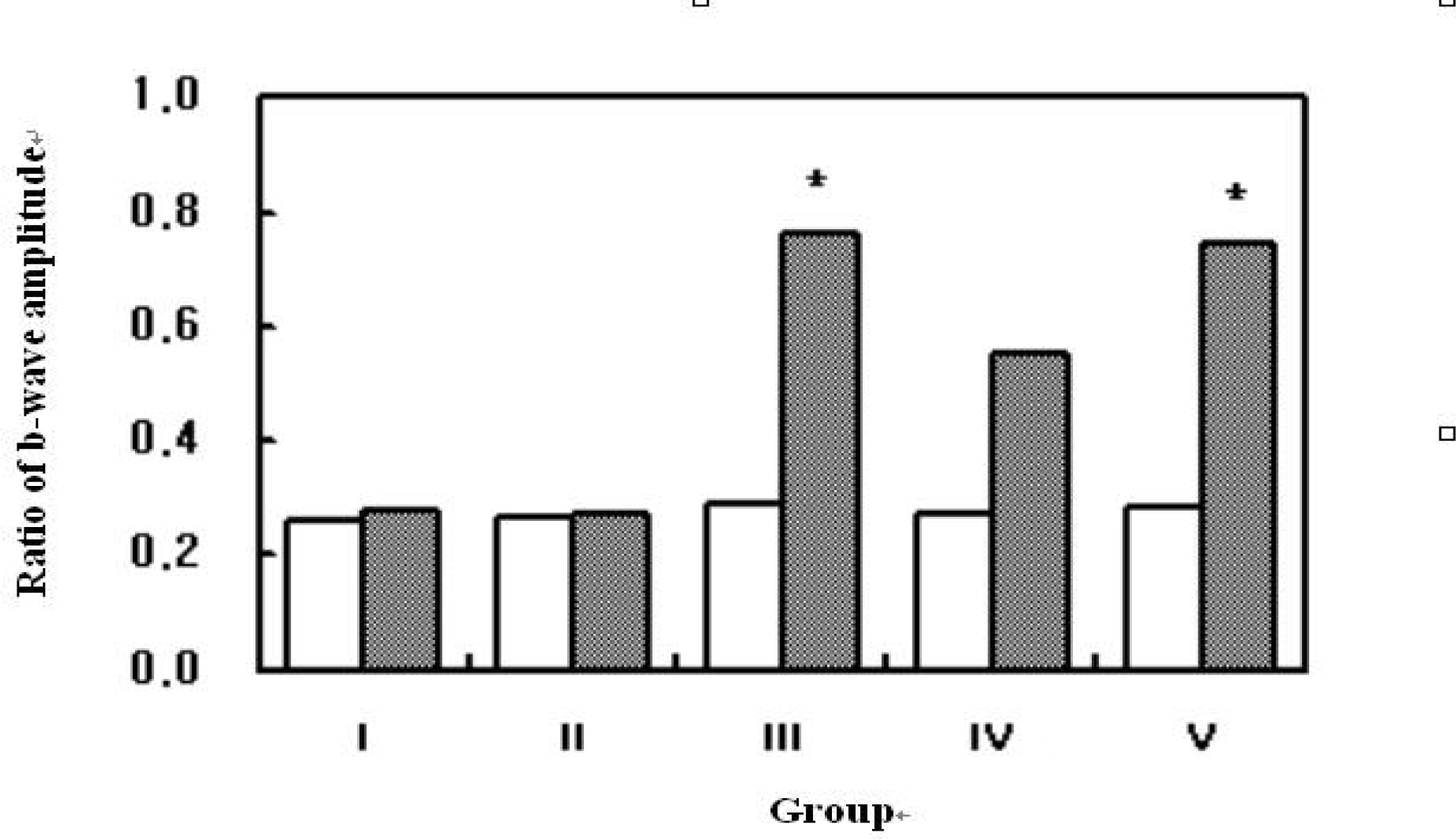 | Figure 3.Ratio of b-wave amplitude (uveitis-induced fellow eye) in each group. (open bars = after induction of uveitis, filled bars = after administration of drugs) *: statistically significant. |
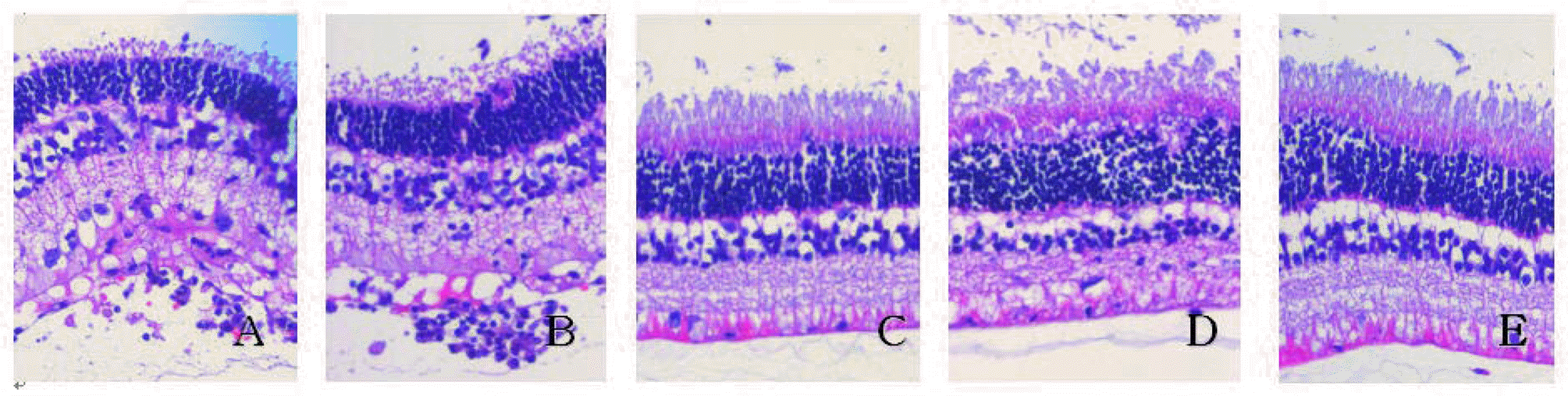 | Figure 4.Histopathologic changes of the retina on day 7 after the administration of drugs.
Note the loss of photoreceptor outer segment and infiltration of inflammatory cells in intravenous BSS-injected eye (A) and intravitreal BSS-injected eye (B). In intravitreal infliximab (1 mg/0.1 ml)-injected eye (D), some loss of photoreceptor outer segment is also observed. Otherwise retinal tissue architectures are well preserved in intravenous infliximab (5 mg/kg)-injected eye (C) and intravitreal infliximab (100 pig/0.1 ml)-injected eye (E).
(E) Intravitreal infliximab (100 gg/0.1 ml)-injected eye. |
Table 1.
Clinical grading of experiment uveitis
Table 2.
Histopathological grading of experimental uveitis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download