Abstract
Purpose
To identify the localization of indoleamine 2,3-dioxygenase (IDO) in human corneal cells and to evaluate its ability to act as a local immunosuppressive factor.
Methods
The expression profile of IDO was obtained with RT-PCR and Western blot of in a primary culture of human corneal cells (fibroblasts, epithelial cells and endothelial cells). In order to investigate the immunosuppressive function of IDO, immune cells were cultured in a human corneal cell-conditioned medium, and their prolifleration was identified by the MIT assay. Moreover, apoptotic effects of IDO in immune cells treated with IFN-Y were also investigated with apoptosis ELISA.
Results
Among the three different types of human corneal cells analyzed, mRNA and protein expression of IDO was observed only in human comeal fibroblasts. Immune cells cultured in a human comeal fibroblast-conditioned medium showed inhibited proliferation Moreover, IFN-Y-induced expression of IDO significantly enhanced apoptotic ability in a dose-depandant manner.
Go to : 
References
1. Higuchi K, Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967; 120:397–403.

2. Dai W, Gupta SL. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-Y. Upstream control region discriminates between interferon-Y and interferon-Q. J Biol Chem. 1990; 265:19871–7.
3. Carlin JM, Borden EC, Sondel PM, et al. Interferon-inuced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol. 1989; 45:29–34.
4. Hwu P, Du MX, Lapointe R, et al. Indoleamine 2.3-dioxygenase production by human dendritc cells results in the inhibition of T cell proliferation. J Immunol. 2000; 164:3596–9.
5. Taylor MW, Feng GS. Relationship between interferon-Y, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991; 5:2516–22.
6. Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2.3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978; 75:3998–4000.

7. Bianchi M, Bertini R, Ghezzi P. Induction of indoleamine 2.3-dioxygenase by interferon in mice: A study with different recombinant interferons and various cytokines. Biochem Biophys Res Commun. 1988; 152:237–42.
8. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998; 281:1191–3.

9. Munn DH, Shafizadeh E, Attwood JT, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999; 189:1363–72.

10. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999; 20:469–73.

11. Li Y, Tredget EE, Kilani RT, et al. Expression of indoleamine 2.3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Invest Dermatol. 2004; 122:953–64.

12. Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003; 9:1269–74.

13. Mellor AL, Keskin DB, Johnson T, et al. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell resoponses. J Immunol. 2002; 168:3771–6.
14. Friberg M, Jennings R, Alsarraj M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cel evasion of T cell-mediated rejection. Int J Cancer. 2002; 101:151–5.
15. Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000; 41:2506–13.
16. Larkin DFP, Beutelspacher SC, George AJT. Expression and function of indoleamine 2,3-dioxygenase in murine cornea. Invest Ophthalmol Vis Sci (ARVO E-Abstract). 2005; 46:3587.
17. Lee GK, Park HJ, Macleod M, et al. Tryptophan deprivation ensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002; 107:452–60.
18. Morita T, Saito K, Takermura M, et al. 3-hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-gamma. Ann Clin Biochem. 2001; 38:242–51.
Go to : 
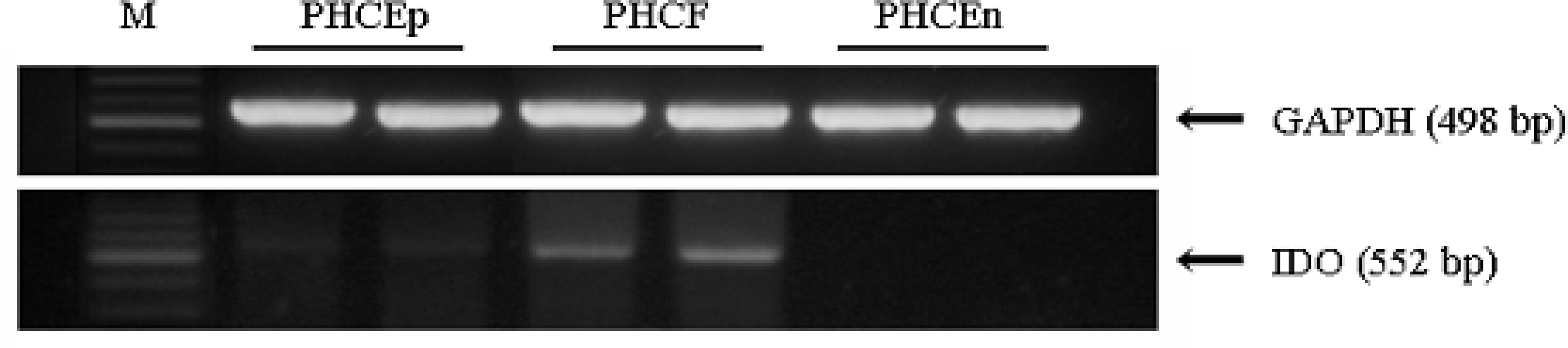 | Figure 1.Expression of IDO in human corneal cells. RT-PCR was performed to identify the expression of IDO in primary cultured human corneal cells. Among three different types of human corneal cells, PHCEn did not express IDO. IDO expression was observed in PHCEp and PHCF. PHCF showed significantly higher expression. |
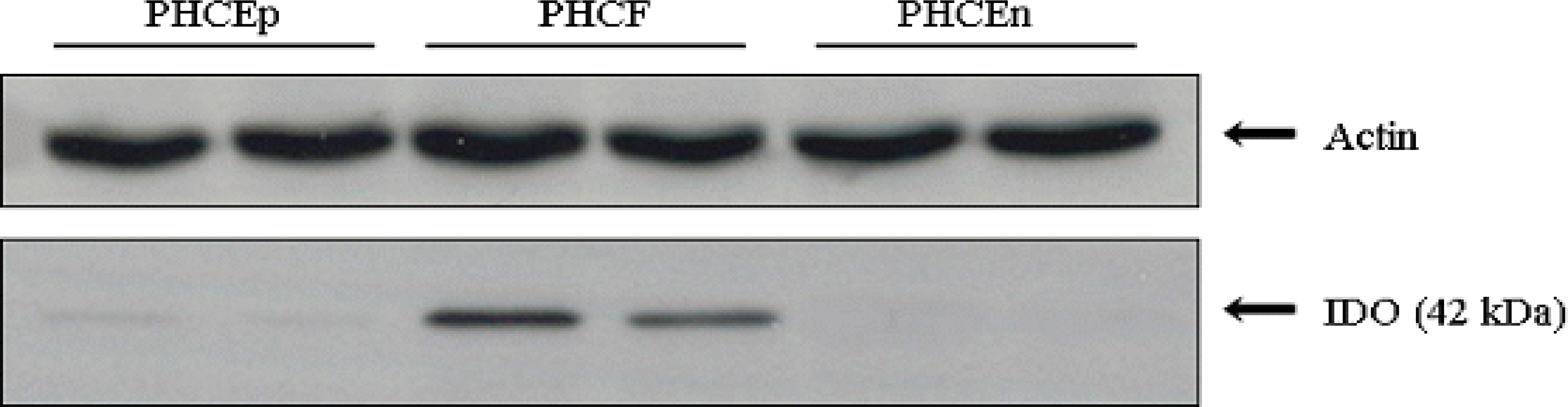 | Figure 2.Expression of IDO in human corneal cells. Western blotting performed to identify the expression of IDO in primary cultured human corneal cells. Among three different types of human corneal cells, PHCEn did not express IDO. PHCF showed significantly higher IDO expression, and PHCEp also showed IDO expression. |
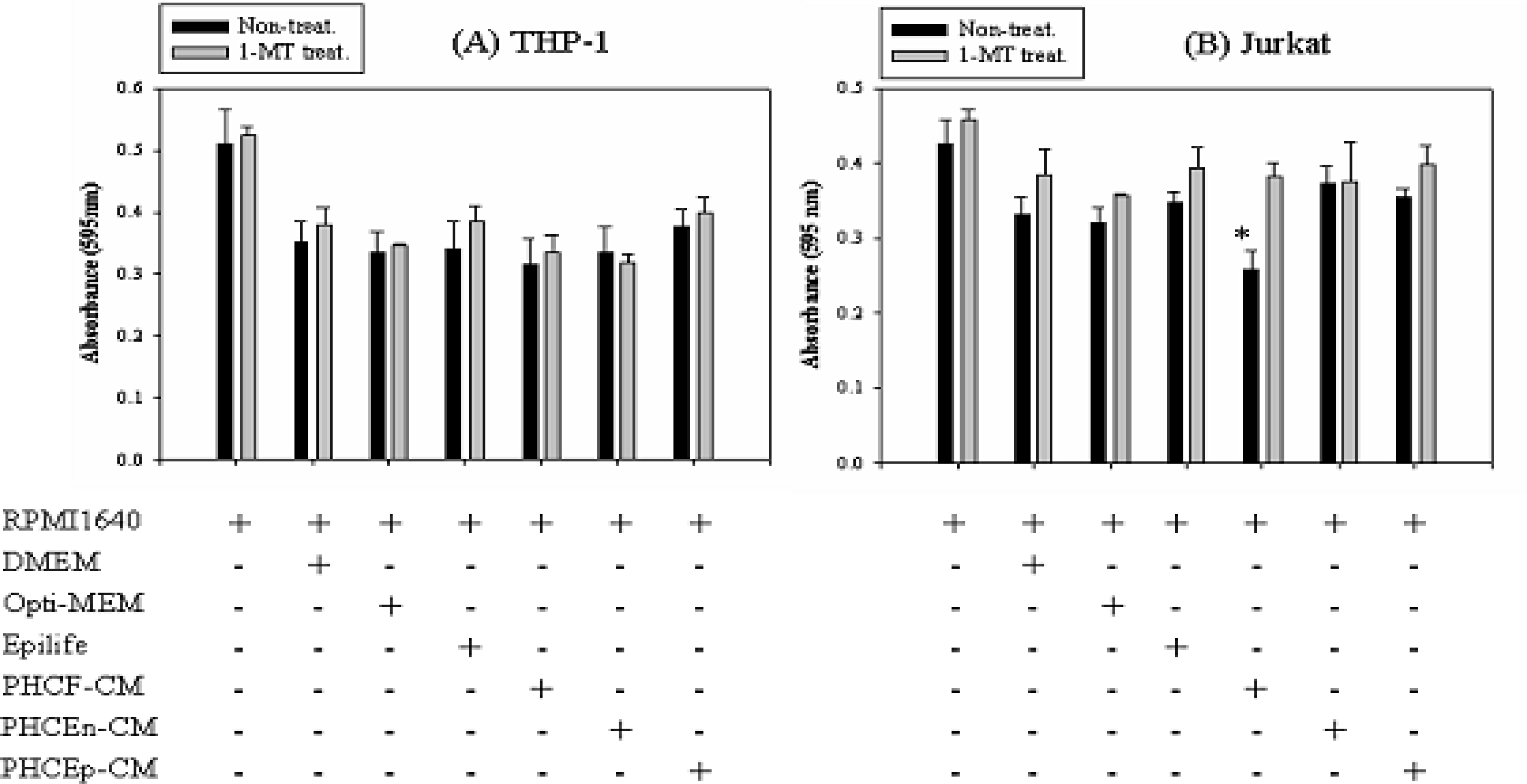 | Figure 3.Proliferation of immune cells cultured in human corneal cells conditioned medium. In order to investigate the effects of IDO expressed by human corneal cells on the proliferation of immune cells, an MTT cell proliferation assay was performed. Human immune cells, THP-1 and Jurkat cells, were cultured in three different types of human corneal cells conditioned medium. To remove the effects of the medium, immune cells were cultured in human corneal cell-defined medium. (A) Although the human monocytes, THP-1, showed decreased proliferation compared with the control (cultured in RPMI1640), there were no effects of the conditioned medium. (B) Human T-lymphocytes (Jurkat cells) cultured in defined medium for human corneal cells showed slightly decreased proliferation. PHCEn-conditioned medium did not show any effects on proliferation of the Jurkat cells. However, PHCF and PHCEp-conditioned medium showed decreased proliferation of Jurkat cells (*P>0.05). Data represent the mean(SD of three separate experiments. |
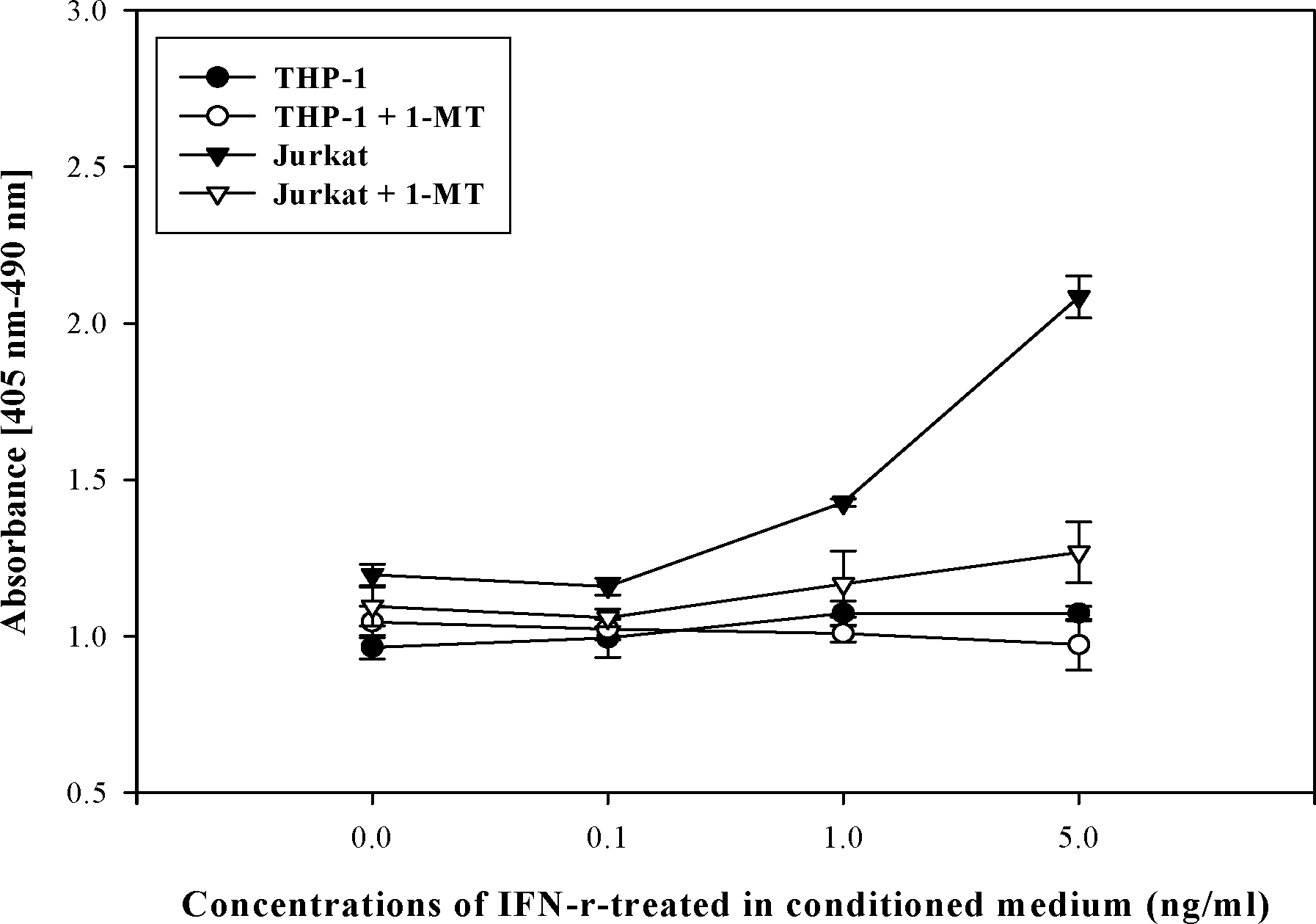 | Figure 4.Apoptosis of immune cells cultured in IFN-V -treated PHCF-conditioned medium. Immune cells were cultured in IFN-v-treated PHCF-conditioned medium for 24 hrs, and cellular apoptosis was measured by ELISA. The THP-1 culture was not affected by IFN-V treatment. However, Jurkat cells showed increased apoptosis in 1.0 and 5.0 ng/ml IFN-Y-treated conditioned medium. IDO neutralizer, 1-MT, treatment showed recovered apoptosis. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download