Abstract
Purpose
To evaluate the demographic and clinical characteristics of bimatoprost-induced periocular skin hyperpigmentation.
Methods
The chart analyses of 16 patients in whom cosmetically noticeable periocular skin hyperpigmentation developed after starting bimatoprost therapy were reviewed. Data collated included age, medication history, dates of starting and stopping bimatoprost treatment, and the subjective assessment of the periocular hyperpigmentation at initial detection as well as follow-up visits.
Periocular hyperpigmentation was graded using an arbitrary scale from 0 to 3. The number of days to the onset of hyperpigmentation and to pigment resolution was determined and their associations to demographic and other clinical parameters were analyzed.
Results
Patients had variable grades of periocular hyperpigmentation at presentation (mean, 1.53±0.66). Bimatoprost-induced periocular hyperpigmentation appeared 1-10 (mean, 4.3±2.6) months after initiation of bimatoprost therapy. Resolution of skin hyperpigmentation was noted 2-10(mean 6.8±1.9) months after stopping bimatoprost treatment. There was a minor correlation(R=+0.19) between the number of days to resolution of hyperpigmentation and the number of days when bimatoprost was used. At 12 months after stopping bimatoprost treatment, 12 of the 13 patients had complete resolution of periocular hyperpigmentation. However, weak hyperpigmentation remained in one patient.
Go to : 
References
1. Woodward DF, Krauss AHP, Chen J, et al. Pharmacological characterization of a novel antiglaucoma agent, bimatoprost (AGN 192024). J Pharmacol Exp Ther. 2003; 305:772–85.

3. Wand M, Ritch R, Isbey EK Jr, Zimmerman TJ. Latanoprost and periocular skin color changes. Arch Ophthalmol. 2001; 119:614–5.
4. Lee JW, Kim DY, Lee YK. Two Cases of Deepening of the Upper Lid Sulcus from Topical Bimatoprost Therapy. J Korean Ophthalmol Soc. 2007; 48:332–6.
5. Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997; 124:544–7.

6. Herndon LW, Williams RD, Wand M, Asrani S. Increased periocular pigmentation with ocular hypotensive lipid use in African Americans. Am J Ophthalmol. 2003; 135:713–5.

7. Grierson I, Jonsson M, Cracknell K. Latanoprost and Pigmentation. Jpn J Ophthalmol. 2004; 48:602–12.

8. Woodward DF, Krauss AH, Chen J, et al. The pharmacology of bimatoprost (Lumigan). Surv Ophthalmol. 2001; 45:337–45.

9. Tomita Y, Maeda K, Tagami H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, urticaria pigmentosa and sunburn. Dermatologica. 1989; 179:49–53.

10. Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001; 14:236–42.

11. Kapur R, Osmanovic S, Toyran S, Edward DP. Bimatoprost induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005; 123:1541–6.
12. Kim EC, Kim JW. Effect of Latanoprost on the Cultured Human Uveal Melanocytes. J Korean Ophthalmol Soc. 2001; 42:893–97.
13. Marco C, Francesco O, Sergio C, Lucia T. Prevention of dermatologic side effects of bimatoprost 0.03% topical therapy. Am J Ophthalmol. 2006; 142:1059–60.
Go to : 
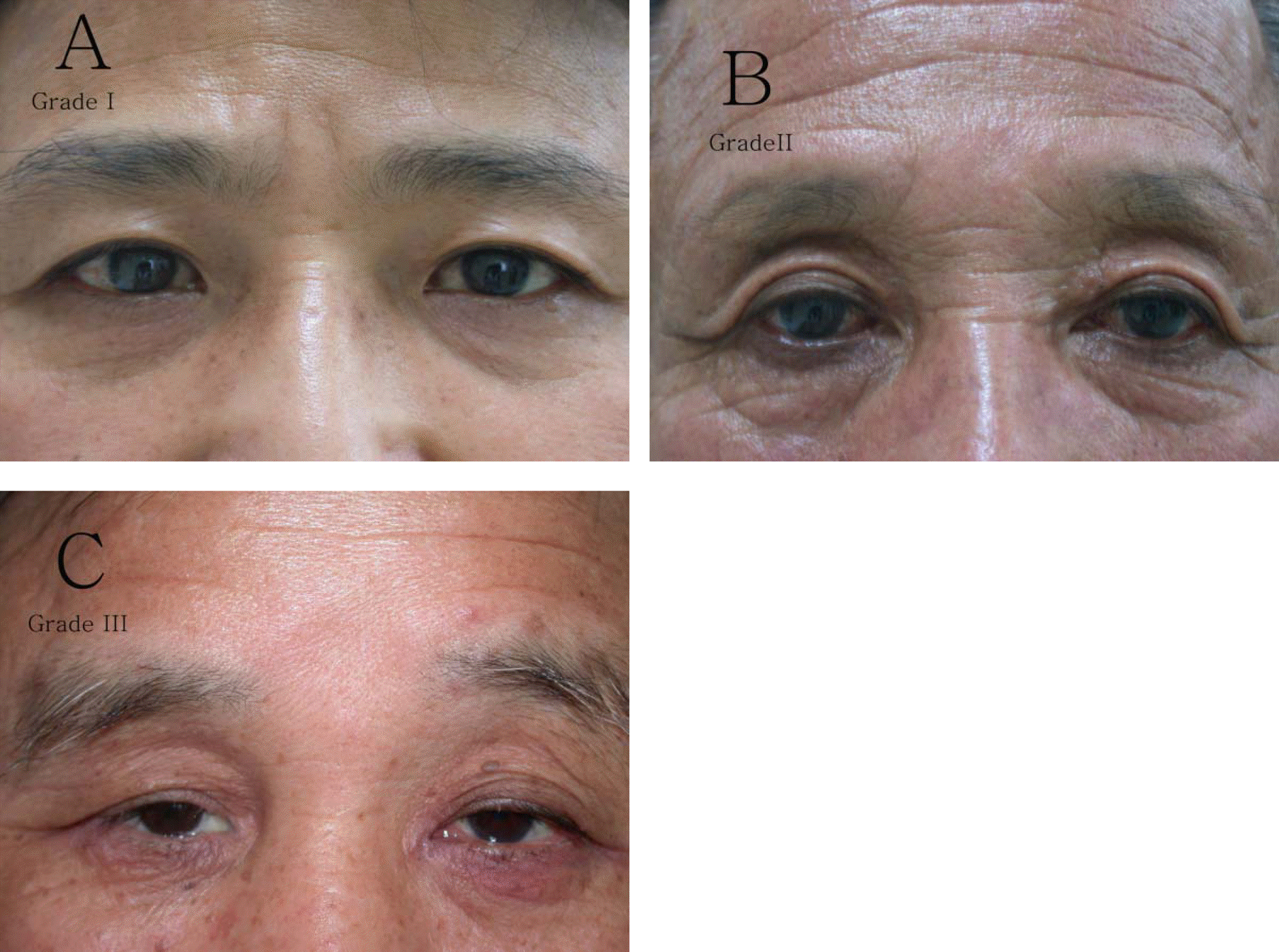 | Figure 1.Photographs of a patient with periocular skin pigmentation. (A) Grade 1 pigmentation with periocular skin pigmentation mainly in the lower eyelids. (B) Grade 2 pigmentation located in both the upper and lower eyelids. (C) Grade 3 pigmentation with marked periocular skin pigmentation and erythema involving both the upper and lower eyelids. |
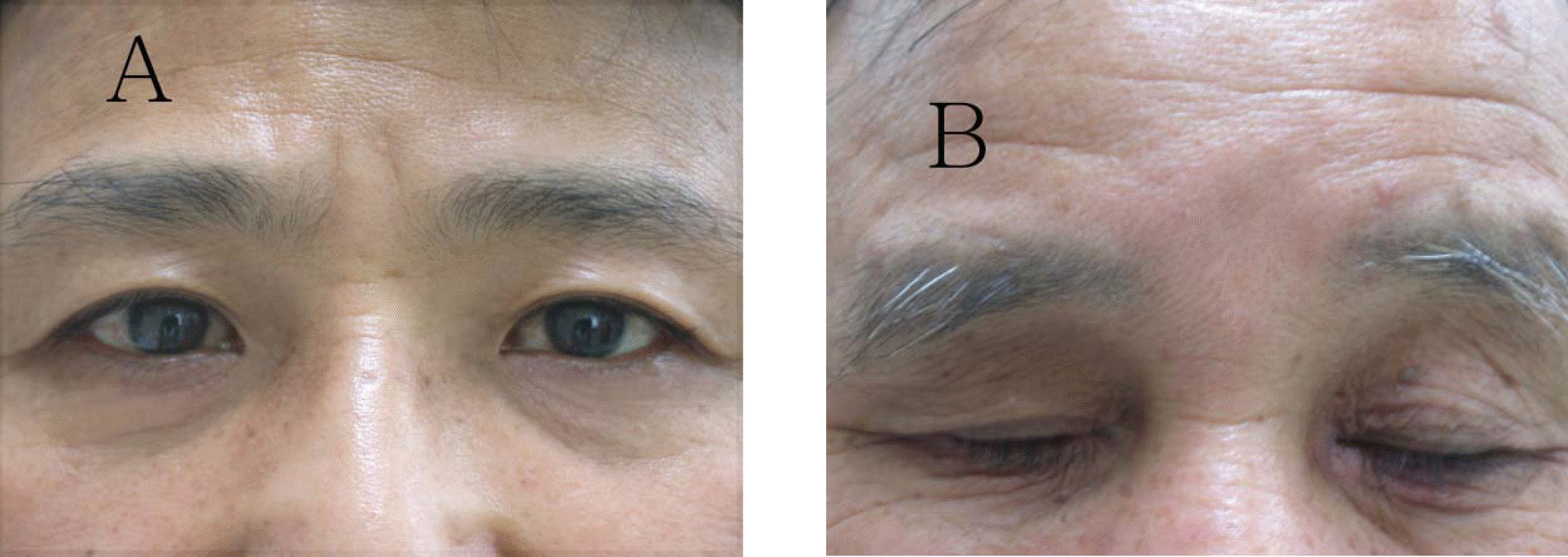 | Figure 2.Resolution of periocular hyperpigmentation (A) 7 months after discontinuation of bimatoprost. (B) Improved grade 3 pigmentation and erythema at 6 months after discontinuation. |
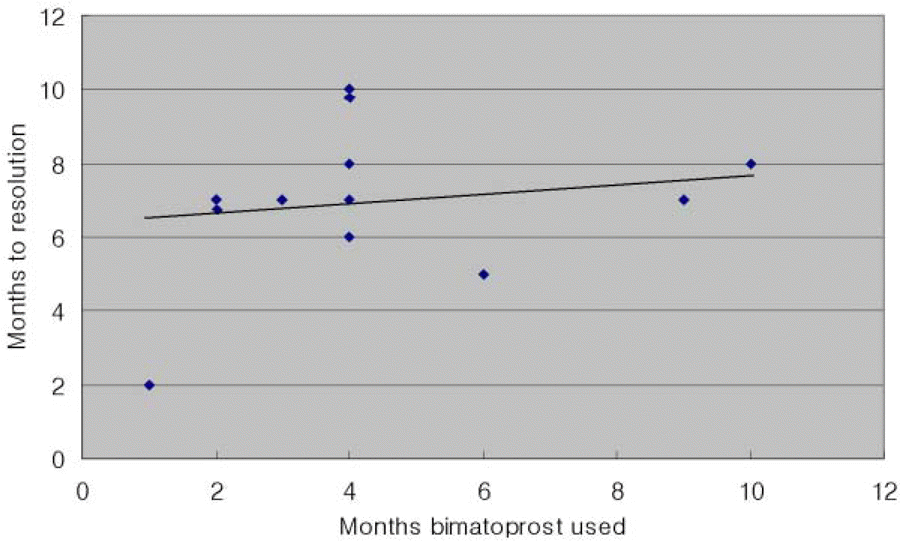 | Figure 3.Scattergram of 12 patients completely resolved pigmentation showing the relationship between the number of months to resolution of hyperpigmentation and the number of months of bimatoprost treatment (R=+0.19, a=0.05). The best-fit line portrays the weakly positive correlation. |
Table 1.
Clinical characteristics of bimatoprost-induced periocular skin pigmentation
| Case | Age/Gender | Months bimatoprost used | Pigmentation grade | Months to pigmentation resolution | Bimatoprost replacement drug |
|---|---|---|---|---|---|
| 1 | 56/M | 3 | 2 | No resolution | TDFC∗, Brimonidine |
| 2 | 65/F | 1 | 1 | 2 | TDFC |
| 3 | 72/F | 2 | 1 | 7 | TDFC |
| 4 | 17/M | 2 | 2 | 7 | Timolol |
| 5 | 46/F | 3 | 1 | 7 | TDFC, Brimonidine |
| 6 | 55/F | 4 | 1 | 6 | TDFC |
| 7 | 67/M | 4 | 3 | 7 | Timolol |
| 8 | 68/M | 4 | 2 | 8 | Timolol |
| 9 | 64/F | 4 | 1 | 8 | TDFC |
| 10 | 70/F | 4 | 2 | 10 | TDFC |
| 11 | 73/M | 6 | 1 | 5 | TDFC, Brimonidine |
| 12 | 68/F | 8 | 1 | 7 | TDFC, Brimonidine |
| 13 | 62/M | 9 | 2 | 8 | TDFC |
| Mean (SD†) | 60.2 (15.0) | 4.3 (2.6) | 1.53 (0.66) | 6.8 (1.9) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download