Abstract
There are currently over 5,000-known species of mushrooms worldwide. Only 20–25% of mushrooms have been named, and 3% of these are poisonous. More than 95% of mushroom poisoning cases occur due to difficulties associated with the identification of mushroom species. Most of the fatal mushroom poisoning cases recorded to date have been related to the Amanita species. Until now, a case of fatal poisoning caused by Macrolepiota neomastoidea (M. neomastoidea) has not been reported in Asia. A 57-year-old male patient was admitted to the emergency room with nausea, vomiting, diarrhea, and abdominal pain. He reported ingesting wild mushrooms with his mother and sister about 2 days ago. His mother and sister were treated with only supportive care, but he was admitted to the intensive care unit and underwent liver transplantation due to acute liver failure. We are reporting a case of fatal M. neomastoidea intoxication from wild mushrooms, a rare case of mushroom poisoning.
There are currently over 5,000-known species of mushrooms worldwide.1 Among them, approximately 100 species are considered as toxic.2 More than 95% of mushroom poisoning cases occur due to difficulties in correctly identifying the mushroom species.3 Eating wild mushrooms can cause mushroom poisoning, which can even lead to death in severe cases. We experienced a case of three family members who accidently ate Macrolepiota neomastoidea (M. neomastoidea).
A 57-year-old male patient with remote history of colon cancer, for which he had undergone a right hemicolectomy, presented to the emergency room with nausea, vomiting, diarrhea, and abdominal pain. Two days prior to admission, he ate M. neomastoidea with his sister and mother, as a result of misidentification for Macrolepiota procera, a type of edible mushroom commonly found in mountains (Fig. 1). He experienced watery diarrhea by more than 20 times and anorexia within 24 hours after indigestion. He did not consume alcohol and any medication. He was fully oriented and vital signs were normal. Icteric sclera were observed. His abdomen was soft with epigastric tenderness with increased bowel sounds. There were no appreciable hepatomegaly and ascites. Laboratory studies were as follows: aspartate aminotransferase, 2,156 IU/L; alanine aminotransferase, 2,555 IU/L; total bilirubin, 3.2 mg/dL; direct bilirubin, 2.3 mg/dL; blood urea nitrogen, 31 mg/dL; creatinine 1.6 mg/dL; lactate dehydrogenase, 2,129 IU/L; prothrombin time 27.1 seconds; and international normalized ratio 2.57. Hepatitis B surface antigen, anti- hepatitis C virus and immunoglobulin M hepatitis A virus were negative. Moreover, other laboratory findings, including complete blood count and alkaline phosphatase levels, were within normal range. Abdominal computed tomography revealed no abnormal findings in the liver.
He was then admitted to the general ward and rehydrated via intravenous administration with 10% dextrose fluid mixed L-aspartic acid L-ornithine 5 g and multivitamins. Complete blood count, biochemistry profile, coagulation function test, and blood gas monitoring were performed daily (Table 1). Despite the best medical supportive care, coagulation function and biochemical laboratory findings began to worsen. On hospital day 3, he started complaining of abdominal discomfort and grew lethargic. He showed worsening liver function and deep drowsy mentality with Model for End-stage Liver Disease score of 34 points. He was transferred to the intensive care unit. We decided to perform a liver transplantation. On hospital day 4, he had deceased donor liver transplantation due to acute hepatic failure. His liver weight was approximately 930 g, which is less than the weight of a normal liver. Gross finding of the liver was discoloration to reddish-green by diffuse cholestasis (Fig. 2). Histopathologic findings showed fulminant hepatitis with massive necrosis of hepatocytes, steatosis, diffuse inflammation, cholestatsis, and bile ductular proliferation (Fig. 3). Symptoms and blood laboratory findings have improved after liver transplantation. At 19 days following liver transplantation, he was discharged.
His mother and sister also indigested the same mushroom. His mother did not present any symptom and abnormal laboratory findings; however, his sister presented the same symptoms as the male patient. Laboratory findings revealed elevated aspartate aminotransferase (2,555 IU/L), alanine aminotransferase (4,298 IU/L), lactate dehydrogenase (4,882 IU/L), and prothrombin time international normalized ratio 1.34. Other laboratory findings were normal. She was fortunately improved with only medical supportive care. On hospital day 7, coagulopathy and liver function had improved and symptoms disappeared.
There are over 5,000-known species of mushrooms worldwide; however, among these, only 20–25% have been named, and only about 100 species are considered poisonous.12 Most patients ingesting toxic mushrooms experience no toxic effects or only experience mild-to-moderate symptoms. However, a few patients experience serious toxicity, such as hepatic failure, renal failure, and pancreatitis.4 According to the Korea Forest Service, 216 cases of mushroom intoxication and 15 deaths have been reported in Korea in the last 10 years. The major cause of death was hepatic failure from ingesting toxic mushrooms.
Such cases typically result from ingesting toxic mushrooms as a result of misidentification of the mushroom with similar morphologic features.3 In our case, the three family members mistakenly thought they were eating an edible mushroom with a similar morphology. Macrolepiota procera is an edible mushroom; the cap is flat, with chocolate-brown colored flakes that remain on the upper surface of the cap without any boundary between the cap and the strip (Fig. 1A). However, in M. neomastoidea, which is a toxic mushroom, the color changes to reddish brown when its fruit body is rubbed (Fig. 1B). This can help distinguish between the two mushrooms.
Mushroom poisoning can be divided into seven categories, depending on the type of toxin: amatoxin, gytomitrin, coprine, muscarine, ivotenic acid-muscimol, psilocybin-psilocin, and gastrointestinal irritants.5 The majority of cases of fatal toxic mushroom poisoning have been induced by amatoxin-containing mushroom, such as Amanita, Galerina, and Lepiota spp., and only a few cases have been caused by other toxins.6 Until now, cases of M. neomastoidea poisoning have rarely been reported in Asia. To the best of our knowledge, only one case was reported in Hong Kong, and it showed gastroenteritic toxicities from self-picked M. neomastoidea ingestion.7 However, cases of M. neomastoidea poisoning inducing a serious hepatic toxicity have not been reported in Asia. Therefore, our case with M. neomastoidea-induced fatal hepatic failure requiring liver transplantation is the first in the world.
M. neomastoidea is a toxic mushroom, which is distributed throughout Korea and other East Asian countries. Mushroom poisoning by M. neomastoidea is associated with gastrointestinal symptoms, including abdominal discomfort, vomiting, and profuse diarrhea.8 However, whether M. neomastoidea poisoning causes liver damage and the pathogenesis of liver damage are unknown.
Macrolepiota species and Lepiota species belonging to the Agaricaceae family contain amatoxin. Amatoxin is one of the most common toxins that induce hepatic toxicity.9 Amatoxin, a thermoresistant toxin that enters into the systemic circulation passing through the intestinal epithelium, induces liver damage. Amatoxins interact with RNA polymerase II in the eukaryotic cells, inhibiting the process of transcription. This results in a progressive decrease in messenger RNA, causing deficient protein synthesis, hepatocyte necrosis, and liver damage. We supposed that one of the pathogenesis of hepatic injury by M. neomastoidea is amatoxin. Kim et al. reported that they isolated four chemical constituents of M. neomatoidea that were associated with cytotoxic activities against the human cells; lepiotin A, B, C, and (R)-5-hydroxypyrrolidine-2-one.10 Considering these findings, we assumed that M. neomatoidea can cause hepatotoxicity.
In this case, the patient's mother and sister ingested the same mushroom and were treated by only medical supportive care. However, the patient experienced acute hepatic failure and was treated by liver transplantation. The patient's mother and sister ingested less amounts of the mushroom than the patient. However, the mechanism of M. neomatoidea inducing hepatotoxicity is unknown; it can be either dose-dependent toxicity or idiosyncratic toxicity. Here, we discovered that M. neomastoidea intoxication can widely impact the liver, ranging from mildly elevated liver enzymes to acute hepatic failure requiring liver transplantation.
Figures and Tables
Fig. 2
Gross findings. Liver shows reddish-green discoloration by diffuse cholestasis. The weight is approximately 930 g, which is lesser than that of normal liver.
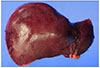
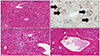
Fig. 3
Histopathologic findings. (A) Massive hepatocellular necrosis is present in hepatocytes. Residual viable hepatocytes show moderate macrovesicular steatosis (H&E, ×20). (B) Bile ductular proliferation (black arrows) is distinctively observed (Immunohistochemistry with anti-cytokeratin18, ×20). (C, D) There is diffuse and remarkable lobular and porto-periportal inflammation (H&E, ×100).
References
1. Gonmori K, Yoshioka N. The examination of mushroom poisonings at Akita University. Leg Med (Tokyo). 2003; 5:Suppl 1. S83–S86.

2. Yardan T, Baydin A, Eden AO, et al. Wild mushroom poisonings in the Middle Black Sea region in Turkey: analyses of 6 years. Hum Exp Toxicol. 2010; 29:767–771.

3. Lin YM, Wang TL. Mushroom poisoning. Ann Disaster Med. 2004; 3:Suppl 1. S8–S11.
4. Mottram AR, Lazio MP, Bryant SM. Lepiota subincarnata J.E. Lange induced fulminant hepatic failure presenting with pancreatitis. J Med Toxicol. 2010; 6:155–157.

5. Sohn CH. Type and treatment of toxic mushroom poisoning in Korea. J Korean Med Assoc. 2015; 58:818–824.

7. Chan CK, Lam HC, Chiu SW, Tse ML, Lau FL. Mushroom poisoning in Hong Kong: a ten-year review. Hong Kong Med J. 2016; 22:124–130.

8. Kim KH, Lee IK, Park KM, Kim WK, Lee KR. Isolation of γ-lactam alkaloids from the Macrolepiota neomastoidea. Bull Korean Chem Soc. 2008; 29:1591–1593.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download