Abstract
Recently, the incidence and prevalence of inflammatory bowel disease (IBD) have been increasing in worldwide, especially in Asian area. IBD is a chronic and progressive disease eventually causing bowel damage. The advance in the treatment of IBD over the past several decades has been achieved with the development of biologics. Furthermore, goals for management of IBD have been evolving from symptom-based management to mucosal healing, which can reduce the surgery rate and hospitalization. To treat the patients with IBD properly, identification of risk factors of patients should be preceded. In addition, the knowledge of several drugs, which are available in current situation is essential. In this review, optimal therapeutic approach with drugs including 5-aminosalicylate, steroid, immunomodulators and anti-TNF antagonists is discussed.
Go to : 
References
1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54. e42;quiz e30.

2. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.

3. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. Longterm complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population-based cohorts. Inflamm Bowel Dis. 2011; 17:471–478.

4. Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012; 18:1356–1363.

5. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017; 15:7–37.

6. Park JJ, Yang SK, Ye BD, et al. Second Korean guidelines for the management of Crohn's disease. Intest Res. 2017; 15:38–67.

7. Choi CH, Moon W, Kim YS, et al. Second Korean guideline for the management of ulcerative colitis]. Korean J Gastroenterol. 2017; 69:1–28.

8. Park JJ, Yang SK, Ye BD, et al. Second Korean guidelines for the management of Crohn's disease. Korean J Gastroenterol. 2017; 69:29–54.

9. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10:CD000543.

10. Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn's disease – a SONIC post hoc analysis. Aliment Pharmacol Ther. 2015; 41:734–746.
11. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.

12. Yarur AJ, Strobel SG, Deshpande AR, Abreu MT. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011; 7:652–659.
13. Armuzzi A, Van Assche G, Reinisch W, et al. Results of the 2nd scientific workshop of the ECCO (IV): therapeutic strategies to enhance intestinal healing in inflammatory bowel disease. J Crohns Colitis. 2012; 6:492–502.

14. Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007; 13:278–283.

15. Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A metaanalysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am J Gastroenterol. 2000; 95:1263–1276.

16. Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and metaanalysis. Am J Gastroenterol. 2012; 107:167–176. author reply 177.

17. Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010; (1):CD004115.

18. van Bodegraven AA, Boer RO, Lourens J, Tuynman HA, Sindram JW. Distribution of mesalazine enemas in active and quiescent ulcerative colitis. Aliment Pharmacol Ther. 1996; 10:327–332.

19. Bebb JR, Scott BB. How effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther. 2004; 20:143–149.

20. Cortot A, Maetz D, Degoutte E, et al. Mesalamine foam enema versus mesalamine liquid enema in active left-sided ulcerative colitis. Am J Gastroenterol. 2008; 103:3106–3114.
21. Hartmann F, Stein J. BudMesa-Study Group. Clinical trial: controlled, open, randomized multicentre study comparing the effects of treatment on quality of life, safety and efficacy of budesonide or mesalazine enemas in active left-sided ulcerative colitis. Aliment Pharmacol Ther. 2010; 32:368–376.

22. Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Effect of extended MMX mesalamine therapy for acute, mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2009; 15:1–8.

23. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006; (2):CD000543.

24. Ye B, van Langenberg DR. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J Gastrointest Pharmacol Ther. 2015; 6:137–144.

25. Pimpo MT, Galletti B, Palumbo G, et al. Mesalazine vanishing time from rectal mucosa following its topical administration. J Crohns Colitis. 2010; 4:102–105.

26. Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and metaanalysis. Am J Gastroenterol. 2011; 106:601–616.

27. Sandborn WJ, Regula J, Feagan BG, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009; 137:1934–1943. e1-e3.

28. Kruis W, Kiudelis G, Rácz I, et al. Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, noninferiority trial. Gut. 2009; 58:233–240.

29. Sandborn WJ, Korzenik J, Lashner B, et al. Once-daily dosing of delayed-release oral mesalamine (400-mg tablet) is as effective as twice-daily dosing for maintenance of remission of ulcerative colitis. Gastroenterology. 2010; 138:1286–1296. 1296.e1-e3.
30. Ogata H, Ohori A, Nishino H, Mizushima S, Hagino A, Hibi T. Comparison of efficacies of once-daily dose multimatrix mesalazine and multiple-dose mesalazine for the maintenance of remission in ulcerative colitis: a randomized, double-blind study. Intest Res. 2017; 15:358–367.

31. Gearry RB, Ajlouni Y, Nandurkar S, Iser JH, Gibson PR. 5-amino-salicylic acid (mesalazine) use in Crohn's disease: a survey of the opinions and practice of Australian gastroenterologists. Inflamm Bowel Dis. 2007; 13:1009–1015.

32. Schoepfer AM, Bortolotti M, Pittet V, et al. The gap between scientific evidence and clinical practice:5-aminosalicylates are frequently used for the treatment of Crohn's disease. Aliment Pharmacol Ther. 2014; 40:930–937.
33. Coward S, Kuenzig ME, Hazlewood G, et al. Comparative effectiveness of mesalamine, sulfasalazine, corticosteroids, and budesonide for the induction of remission in Crohn's disease: a Bayesian network metaanalysis. Inflamm Bowel Dis. 2017; 23:461–472.
34. Ferrante M, Karmiris K, Newnham E, et al. Physician perspectives on unresolved issues in the use of conventional therapy in Crohn's disease: results from an international survey and discussion programme. J Crohns Colitis. 2012; 6:116–131.

35. D'Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011; 106:199–212. quiz 213.
36. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™registry. Am J Gastroenterol. 2012; 107:1409–1422.
37. Jeon SR, Kim WH. Is long-term therapy with thiopurines effective for maintaining remission in patients with moderate-to-severe ulcerative colitis? Intest Res. 2015; 13:191–192.

38. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK. IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015; 13:193–207.

39. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015; (10):CD000067.

40. Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; (9):CD000478.

41. Lee HJ, Yang SK, Kim KJ, et al. The safety and efficacy of azathioprine and 6-mercaptopurine in the treatment of Korean patients with Crohn's disease. Intest Res. 2009; 7:22–31.
42. Lee HS, Park SH, Yang SK, et al. Longterm prognosis of ulcerative colitis and its temporal change between 1977 and 2013: a hospital-based cohort study from Korea. J Crohns Colitis. 2015; 9:147–155.

43. Kim B, Cheon JH, Moon HJ, et al. Crohn's disease prognosis and early immunomodulator therapy: results from the CONNECT study. J Gastroenterol Hepatol. 2016; 31:126–132.

44. Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercap-topurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2013; (4):CD000545.

45. Winter JW, Gaffney D, Shapiro D, et al. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007; 25:1069–1077.

46. Kim JH, Cheon JH, Hong SS, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol. 2010; 44:e242–e248.
47. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.

48. Kirchgesner J, Beaugerie L, Carrat F, et al. Impact on life expectancy of withdrawing thiopurines in patients with Crohn's disease in sustained clinical remission: a lifetime risk-benefit analysis. PLoS One. 2016; 11:e0157191.

49. Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013; 369:754–762.

50. Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap Adv Gastroenterol. 2015; 8:66–82.

51. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.

52. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012; 142:1102–1111.e2.

53. Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014; 13:24–30.

54. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003; 348:601–608.

55. Sofia MA, Rubin DT. Current approaches for optimizing the benefit of biologic therapy in ulcerative colitis. Therap Adv Gastroenterol. 2016; 9:548–559.

56. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.

57. Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn's disease: a systematic review and metaanalysis. J Crohns Colitis. 2014; 8:1632–1641.

Go to : 
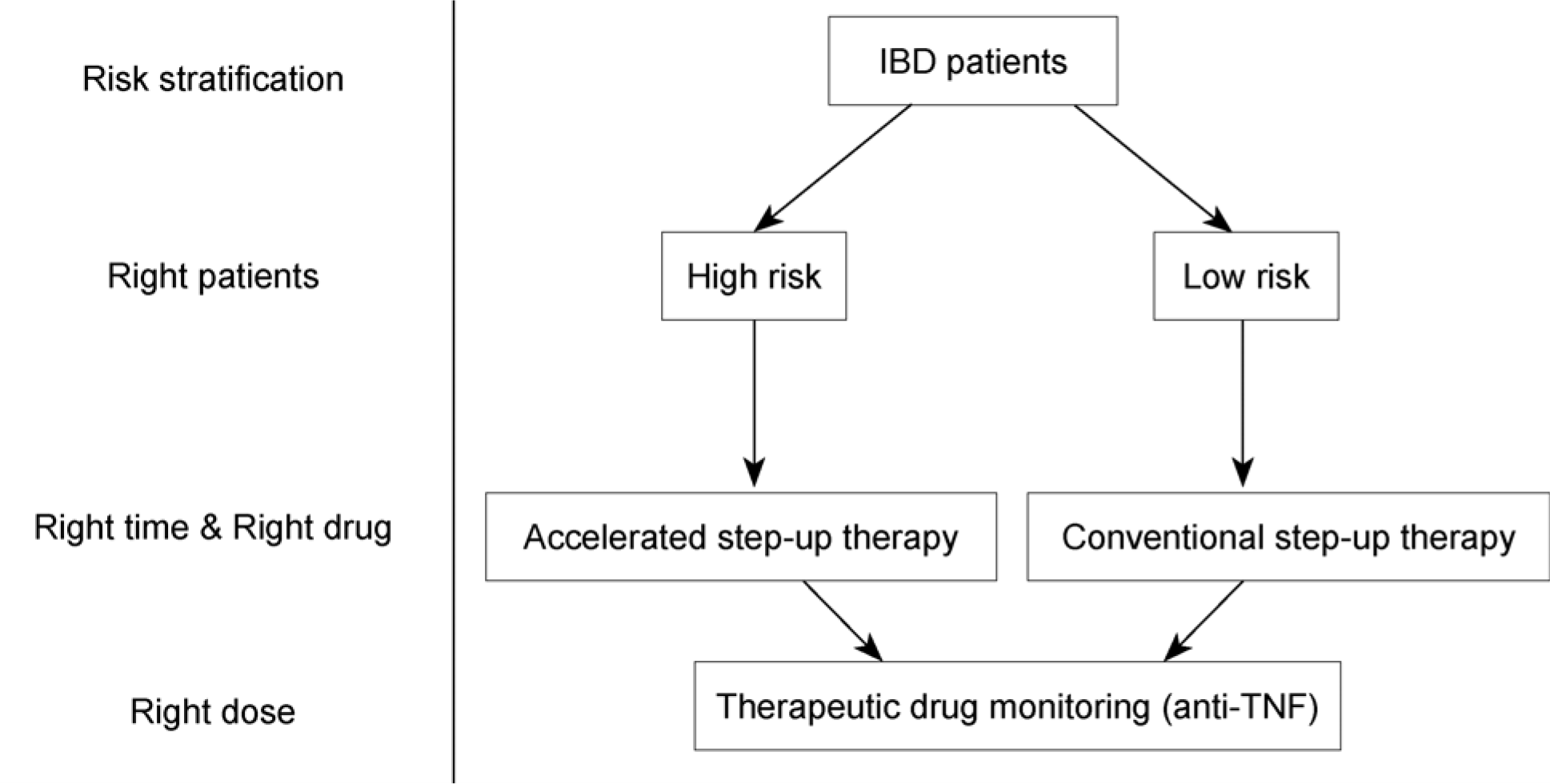 | Fig. 1.Therapeutic approach of personalized medicine in patients with inflammatory bowel disease. IBD, inflammatory bowel disease; anti-TNF, anti-tumor necrosis factor. |
Table 1.
Drugs Which Can Cause the Mucosal Healing of Inflammatory Bowel Disease13
|
Ulcerative colitis |
Crohn's disease |
|||
|---|---|---|---|---|
| Induction | Maintenance | Induction | Maintenance | |
| 5-ASA | + | + | − | − |
| Steroids | + | − | − | − |
| Thiopurines | + | + | + | + |
| Anti-TNF | ++ | ++ | ++ | ++ |
Table 2.
Delivery System and Site of 5-ASA Release




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download