Abstract
It is important to have effective therapeutic strategies and goals in clinical practice and research of inflammatory bowel disease. Conventional end points for clinical trials in Crohn's disease and ulcerative colitis have been based on composite indices, such as the Crohn's Disease Activity Index and the Mayo Clinic Score. Although these indices have been shown to reduce the intestinal injury to some extent, satisfactory results have not been obtained in improving the quality of life of patients. Recently, alternative measures of outcome and definitions of response are being developed beyond symptoms. Mucosal healing as a clinical response and treatment goal has showed better long-term outcomes. Patient-reported outcomes (PROs) are emerging instrument directly created by patient to quantify symptoms. Coprimary realistic treatment ‘target', comprising mucosal healing and PROs, can offer a clinically valid endpoint and can be readily applied in practice compare to existing composite indices. ‘Treat-to-target' algorithm based on mucosal healing and PROs, in which therapy is progressively intensified until a specific personal treatment goal is reached, could improve quality of life of patient by reducing disease-related disability. Furthermore, histologic remission is an area of increased research focus and has the potential to guide treatment decisions in the future.
Go to : 
References
1. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970–2004). Am J Gastroenterol. 2012; 107:1693–1701.

2. Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012; 107:1228–1235.

3. Magro F, Rodrigues A, Vieira AI, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012; 18:573–583.

4. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976; 70:439–444.
5. Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. Gut. 2006; 55(Suppl 1):i1–i15.

6. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483.

7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-amino-salicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987; 317:1625–1629.
8. Travis SP, Stange EF, Lémann M, et al. European evidencebased Consensus on the management of ulcerative colitis: current management. J Crohns Colitis. 2008; 2:24–62.

9. Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.

10. Bernstein CN, Loftus EV Jr, Ng SC, Lakatos PL, Moum B. Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD). Hospitalisations and surgery in Crohn's disease. Gut. 2012; 61:622–629.

11. Frøslie KF, Jahnsen J, Moum BA, Vatn MH. IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.

12. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362:1383–1395.

13. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.

14. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidencebased consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62.

15. Dignass A, Lindsay JO, Sturm A, et al. Second European evidencebased consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012; 6:991–1030.

16. Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011; 140:1817–1826.e2.

17. Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014; 59:829–837.

18. Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009; 15:1190–1198.

19. De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013; 19:2111–2117.
20. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018; 390:2779–2789.

21. Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014; 20:1407–1415.
22. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.
23. US Food and Drug Administration. Gastroenterology regulatory endpoints and the advancement of therapeutics (GREAT II) workshop; October 21–22. 2013. Bethesda, MD.
24. U. S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006; 4:79.

25. Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015; 148:37–51.e1.

26. Sandler RS, Jordan MC, Kupper LL. Development of a Crohn's index for survey research. J Clin Epidemiol. 1988; 41:451–458.

27. Khanna R, Zou G, D'Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Aliment Pharmacol Ther. 2015; 41:77–86.

28. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.

29. Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014; 12:1246–1256.e6.

30. Sandborn WJ, Hanauer S, Van Assche G, et al. Treating beyond symptoms with a view to improving patient outcomes in inflammatory bowel diseases. J Crohns Colitis. 2014; 8:927–935.

31. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015; 13:1042–1050.

32. Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014; 12:929–934.e2.
33. Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2017; 66:50–58.

34. Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014; 8:1582–1597.

35. Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extraintestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.

36. Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991; 32:174–178.

37. Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016; 65:408–414.
38. Gomollón F, Dignass A, Annese V, et al. 3rd European evidencebased consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.

39. Feagan BG. Enhanced algorithm for Crohn's treatment incorporating early combination therapy (REACT2). ClinicalTrials.gov identi-fier: NCT01698307. [Internet]. Bethesda (MD): National Institutes of Health;2012 Oct 2. [updated 2017 Jul 17;cited 2018 Jan 1]. Available from:. http://www.clinicaltrials.gov/ct2/results?term=NCT01698307.
Go to : 
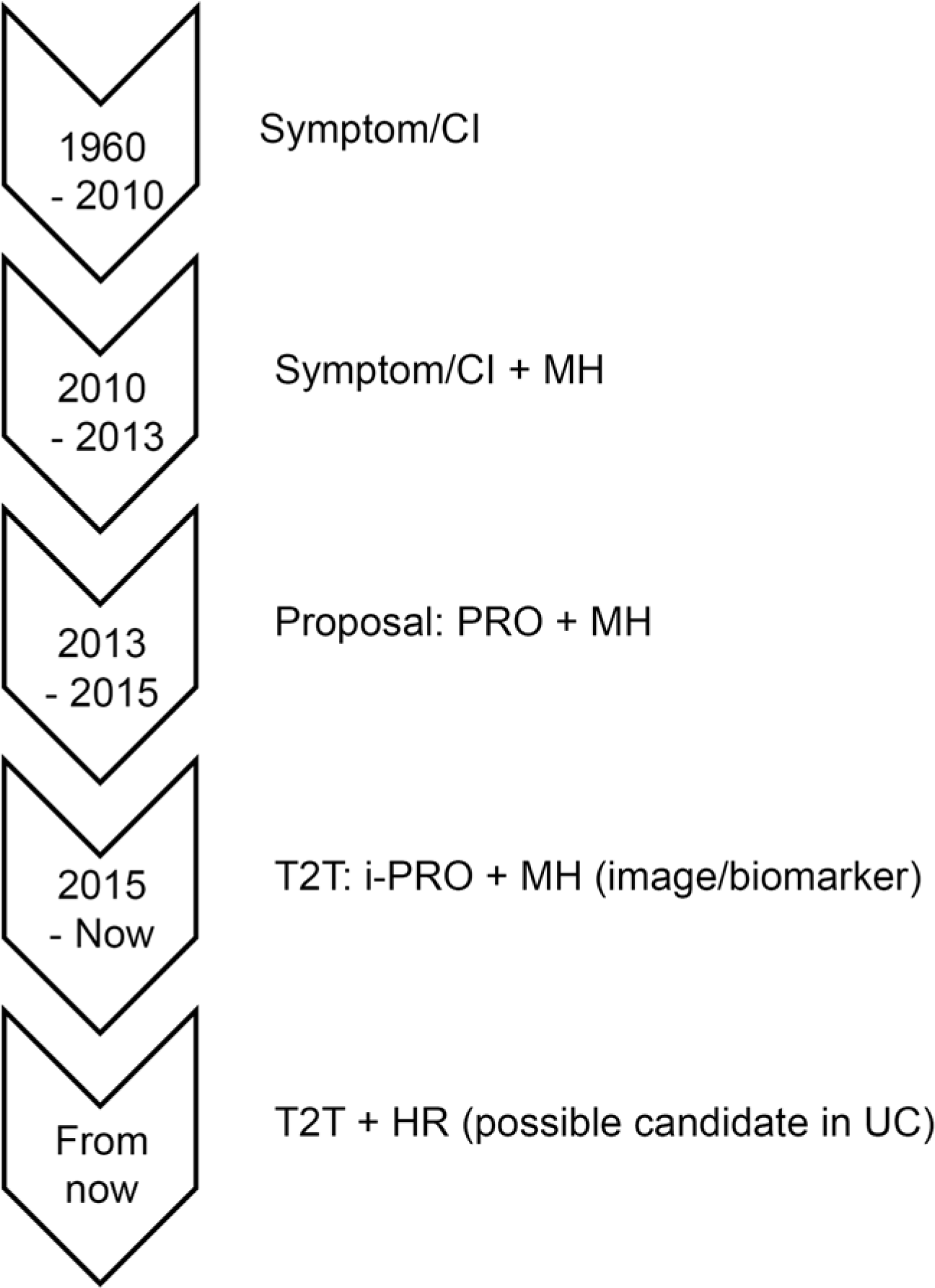 | Fig. 2.Treatment strategies and goals for inflammatory bowel disease over time. CI, composite index; MH, mucosal healing; PRO, patient-reported outcome; i-PRO, interim PRO; HR, histologic remission; T2T, treat-to-target; UC, ulcerative colitis. |
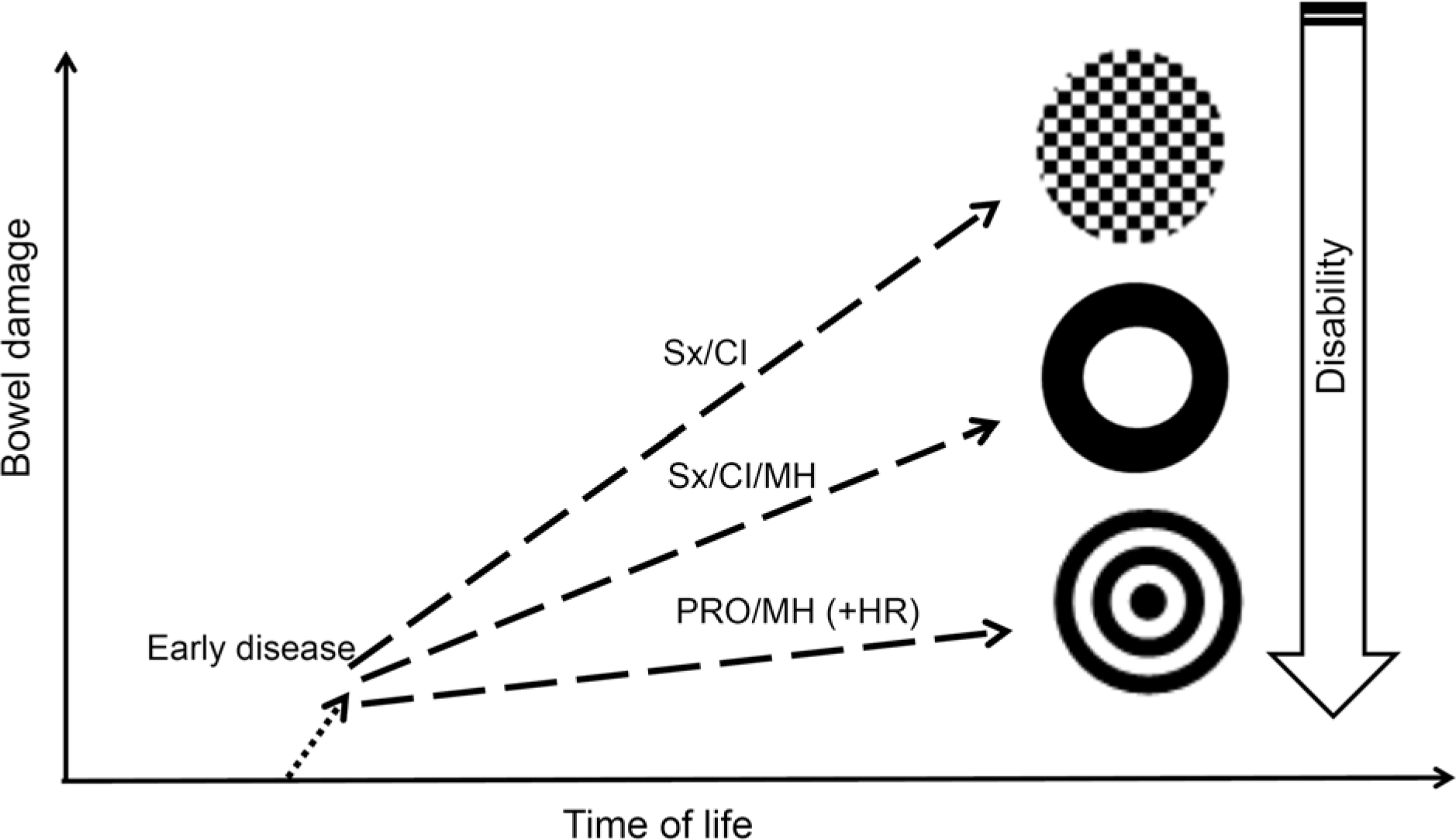 | Fig. 3.Evolving concept of therapeutic “target” in inflammatory bowel disease. Sx, symptom; CI, composite index; MH, mucosal healing; PRO, patient-reported outcome, HR, histologic remission. |
Table 1.
Crohn's Disease Activity Index (CDAI)4
Table 2.
Mayo Clinic Score (MCS)7
A score of 3 for bleeding required patients to have at least 50% of bowel motions accompanied by visible blood and at least one bowel motion with blood alone. MCS (range, 0 to 12); ≤2 and no subscore >1, clinical remission; 3 to 5, mild activity; 6 to 10, moderate activity; and 11 to 12, severe activity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download