Most primary tumors of the liver are hepatocellular carcinoma (HCC); benign tumors are also relatively common. Among the benign tumors, hepatic hemangioma is most common but angiomyolipoma (AML) is relatively rare.1 AML is a benign mesenchymal tumor composed of blood vessels, smooth muscle cells, and a varying amount of fat.2 AML occurs most often in the kidneys, with the liver being the second most frequent site of involvement.3 The proportion of each component vary. The tumors can be categorized according to their predominant component as mixed, lipomatous, myomatous, and angiomatous type. The mixed type is most common, which is composed of epithelioid muscle cells and mixed with islands of fat and abnormal vessels. The myomatous type is more common in the liver than the kidney.4 This rare tumor is usually detected incidentally on imaging studies, such as ultrasonography (US), magnetic resonance imaging (MRI), computed tomography (CT), during the annual health check of healthy people with no underlying liver disease. AML can be exacerbated by estrogens but the precise mechanism is not known.5 The size of hepatic AML varies from 0.1 cm to 40 cm, and its morphology and histology vary.6 The tumors are usually benign, but several cases of malignancy have been also reported. The tumor has also been misdiagnosed as malignant neoplasm, lipoma, sarcoma, or other metastatic neoplasm. The diagnoses are confirmed by a pathologic examination and immunochemical assays with a broad panel of antibodies, including human melanocyte B-45 (HMB-45).
This paper reports a rare case of hepatic AML that contained no adipose tissue, mimicking HCC on the imaging studies, and diagnosed by a surgical tissue biopsy.
A 65-year-old woman, who had taken hormone therapy for 3 years 13 years prior, underwent an abdominal US for annual health check. She had no evidence of tuberous sclerosis clinically. A 1.9×1.5 cm sized liver tumor of S6 was detected by US. Abdominal-pelvic CT and magnetic resonance image (MRI) were performed for a further evaluation. The physical examination showed no abnormal findings. The blood chemistry test revealed the following: hemoglobin, 12.9 g/dL; leukocyte count, 5,980/mm3 with 66.1% neutrophil; platelet count, 200,000/mm3; total bilirubin, 1.0 mg/dL; aspartate aminotransferase, 17 IU/L; and alanine aminotransferase, 8 IU/L. The tumor markers showed 1.6 ng/mL (normal range, 0–10) of α-fetoprotein, 20 mAU/mL (normal range, 0–40) of protein induced by the absence of vitamin K or antagonist-II, and 22 U/mL (normal range, 0–37) of carbohydrate antigen 19-9, and 1.0 ng/mL (normal range, 0–5) of carcinoembryonic antigen. The hepatitis B surface antigen was negative and the surface antibody was positive. The hepatitis C antibody was negative. On US, a 1.9×1.5 cm sized, slightly low echoic oval mass was noted in the anteroinferior aspect of S6 of the liver (Fig. 1). CT revealed a 1.9×1.5 cm sized oval mass with a sharp and smooth margin at the upper anteromedial aspect of S6. The arterial and portal phase showed total high enhancement and the delayed phase revealed iso-density (Fig. 2). On MRI, the mass was observed as homogeneous low signal intensity in T1WI and homogenous high signal intensity in T2WI. On the Gadoxetic acid (Gd-EOB-DTPA) enhanced images, the mass showed early homogeneous high enhancement in 30 seconds, almost iso-signal intensity with liver in the 1 minute image, and homogeneous washout with rim-like high enhancement in the 3 minute delay image (Fig. 3). No focal lesion was observed on the spleen, pancreas, and kidneys. A tumor biopsy was required because a malignancy could not be excluded. On the other hand, there was a possibility of tumor seeding through the biopsy tract, tumor bleeding or tumor rupture during the needle biopsy. Therefore, a decision was made to proceed with surgery. She underwent a right posterior segmentectomy (Fig. 4). The tumor cells were positive for HMB-45 and negative for cytokeratin and hepatocyte paraffin 1. An examination of the intraoperative frozen section indicated a hepatocellular neoplasm and suggested HCC. In the frozen section, the possibility of a hepatocellular neoplastic lesion was considered. The priority of angiomyolipoma, a very rare tumor, was not considered because of the absence of fat tissue and spindle cells were not observed. The tumors were believed to have originated in hepatocytes and suggested the possibility of a hepatocellular adenoma or hepatocellular carcinoma with good differentiation. These two lesions are difficult to distinguish from the permanent sections or immunohistochemical staining as well as frozen sections. Therefore, the frozen section diagnosis indicated the possibility of well differentiated hepatocellular carcinoma.
|
|

|
Fig. 1
On ultrasonography, 1.9×1.5 cm sized, slightly low echoic oval mass was noted in the anteroinferior aspect of S6 of the liver (arrow).
|
|
 Click for larger image Click for larger image |
|
|
|

|
Fig. 2
1.9×1.5 cm sized oval shaped tumor in the upper anteromedial aspect of segment 6 of the liver (arrow). The hepatic arterial phase (A) and portal phase (B) of dynamic-enhanced computed tomography scan show a hyperdense hepatic tumor. The tumor is iso-density in the delayed phase (C).
|
|
 Click for larger image Click for larger image |
|
|
|

|
Fig. 3
Gadoxetic acid (Gd-EOB-DTPA) enhanced dynamic magnetic resonance imaging scan shows intense and early homogeneous enhancement of the tumor (arrow) in 30 seconds (A) and iso-signal intensity at 1 minute after injection (B), and lower signal intensity of the mass compared to the increased signal intensity of the surrounding liver at 3 minutes after injection (C).
|
|
 Click for larger image Click for larger image |
|
|
|
|
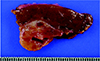
Fig. 4
Gross photograph after segmentectomy of the liver. A well-defined, round, brown tinged grayish mass has a soft to rubbery surface and solid appearance.
|
|
 Click for larger image Click for larger image |
|
On the other hand, the final pathologic diagnosis was the epithelioid myoid type of AML with no adipose tissue component. The tumor was diffusely positive in the HMB-45 immunochemistry stain (Fig. 5), negative to cytokeratin and hepatocyte paraffin 1, which excluded a hepatocellular origin tumor, such as HCC or hepatocellular adenoma. The tumor cells were partially positive for vimentin and the Ki-67 labeling index was 2%. Immunohistochemical staining for SMA and CD34 was not performed because immunohistochemical staining for benign tumors can be reduced. Microscopically, the tumor was composed mainly of an epithelioid myomatous component. An angiomatous component was rarely observed, and no lipomatous component was observed. Multifocal lymphocytic infiltration was noted but no cytologic atypia, necrosis, lymphatic, neural or angioinvasion, and mitotic figures were observed. Therefore, a malignancy could be excluded. After resection of the tumor in the liver, the patient was discharged. After two visits since discharge, the patient was lost to follow-up.
|
|

|
Fig. 5
Histologic and immunohistochemical features of this case. (A) The tumor contains abundant epithelioid cells with nuclear pleomorphism and without a lipomatous component (H&E, ×400). (B) Positive immunostaining for the melanoma marker, HMB-45 (HMB-45, ×400). HMB-45, human melanocyte B-45.
|
|
 Click for larger image Click for larger image |
|
Hepatic AML is usually noted as a solitary hepatic tumor with or without symptoms. The onset of AML is insidious; most patients have no obvious symptoms and are mostly discovered during a routine clinical examination. Some patients may feel discomfort or pain in the upper abdominal region. In the present case, the tumor was discovered incidentally in an annual check-up. Ishak described the first case of hepatic AML in 1976.7 To date, approximately 200 hepatic AMLs have been reported.3 Hepatic AMLs are categorized as mixed (the most common type), lipomatous (≥70% fat), myomatous (≤10% fat), and angiomatous types according to the components of the lipid proportion. The classic mixed type of hepatic AML, including all of the tissue components, is most commonly encountered.8 The fat content within the AML can range from 5% to 90% of the tumor volume in each case.9 This can lead to a misdiagnosis clinically because of the diagnostic difficulties. Usually, the characteristics of imaging studies can help determine the predominant histologic components of AML by their correlation with it. MRI is more sensitive than CT for an evaluation of the fat content, which appears hypointense on the fatty components. On the other hand, a differential diagnosis between hepatic AML and other hepatic fatty tumors, such as fat-containing hepatic adenomas or HCC with fatty metamorphosis, is difficult. Furthermore, it is also difficult to exclude a malignancy or adenoma in the case of the slight presence of fatty tissue and the relatively high presence of vascularity. A tumor containing little fat is shown as homogeneous arterial enhancement with washout to isointensity on the portal venous phase. Wang et al.10 reported 9 cases of AML mimicking HCC. On their report, 6 cases were diagnosed as HCC on MRI before the resection. Five of the 9 cases were the myomatous predominant subtype, which showed similar MRI findings to those in the present case.10 In the past, this AML has been considered to be entirely benign but several reports have shown that this type of tumor can be malignant with evidence of recurrence.11, 12, 13 fluorodeoxyglucose positron emission tomography (FDG PET)/CT is a useful tool for detecting malignant transformations.14 A few studies have reported that the FDG PET/CT findings in patients with hepatic AML showed low FDG uptake in the tumor. On the other hand, in the case of intratumoral hemorrhage, the increased FDG uptake can be shown around the hemorrhage due to hemosiderin-laden macrophages.15 In the present case, FDG PET/CT was not performed. Although some imaging features may suggest AML, a definite diagnosis can only be made by a tissue biopsy.
In conclusion, hepatic AML is a rare benign mesenchymal tumor. Hepatic AML with minimal adipose tissue is rarely reported,16, 17 and hepatic AML without any adipose tissue is extremely rare. This paper reports an unusual case of hepatic AML without adipose tissue, mimicking HCC on the imaging studies, which was finally diagnosed by a surgical resection.
Conflict of interest:None.
|
|
| 1. |
Yeh CN, Chen MF, Hung CF, Chen TC, Chao TC. Angiomyolipoma of the liver. J Surg Oncol 2001;77:195–200.
|
|
| 2. |
Xu AM, Zhang SH, Zheng JM, Zheng WQ, Wu MC. Pathological and molecular analysis of sporadic hepatic angiomyolipoma. Hum Pathol 2006;37:735–741.
|
|
| 3. |
Petrolla AA, Xin W. Hepatic angiomyolipoma. Arch Pathol Lab Med 2008;132:1679–1682.
|
|
| 4. |
Liu Y, Wang J, Lin XY, Xu HT, Qiu XS, Wang EH. Inflammatory angiomyolipoma of the liver: a rare hepatic tumor. Diagn Pathol 2012;7:122.
|
|
| 5. |
Clements D, Asprey SL, McCulloch TA, Morris TA, Watson SA, Johnson SR. Analysis of the oestrogen response in an angiomyolipoma derived xenograft model. Endocr Relat Cancer 2009;16:59–72.
|
|
| 6. |
Butte JM, Do RK, Shia J, et al. Liver angiomyolipomas: a clinical, radiologic, and pathologic analysis of 22 patients from a single center. Surgery 2011;150:557–567.
|
|
| 7. |
Ishak KG. Mesenchymal tumor of the liver. In: Okuda K, Peters RL, editors. Hepatocellular Carcinoma. New York: John Wiley; 1976. pp. 247-307.
|
|
| 8. |
Wang SN, Tsai KB, Lee KT. Hepatic angiomyolipoma with trace amounts of fat: a case report and literature review. J Clin Pathol 2006;59:1196–1199.
|
|
| 9. |
Nonomura A, Mizukami Y, Kadoya M. Angiomyolipoma of the liver: a collective review. J Gastroenterol 1994;29:95–105.
|
|
| 10. |
Wang CP, Li HY, Wang H, et al. Hepatic angiomyolipoma mimicking hepatocellular carcinoma: magnetic resonance imaging and clinical pathological characteristics in 9 cases. Medicine (Baltimore) 2014;93:e194
|
|
| 11. |
Dalle I, Sciot R, de Vos R, et al. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology 2000;36:443–450.
|
|
| 12. |
Croquet V, Pilette C, Aubé C, et al. Late recurrence of a hepatic angiomyolipoma. Eur J Gastroenterol Hepatol 2000;12:579–582.
|
|
| 13. |
Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol 2008;32:793–798.
|
|
| 14. |
Macheda M, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 2005;202:654–662.
|
|
| 15. |
Takanami K, Kaneta T, Hitachi S, et al. F-18 FDG PET/CT findings in two patients with hepatic angiomyolipoma with and without intratumoral hemorrhage. Clin Nucl Med 2010;35:18–21.
|
|
| 16. |
Lee SJ, Kim SY, Kim KW, et al. hepatic angiomyolipoma with minimal fat, mimicking hepatocellular carcinoma. Clin Mol Hepatol 2012;18:330–335.
|
|
| 17. |
Hwang I, Yu E, Cho KJ. Hepatic angiomyolipoma with variable histologic features: 8 cases resembling hepatocellular carcinoma or inflammatory pseudotumor. Korean J Gastroenterol 2012;60:242–248.
|
|




 ePub
ePub Citation
Citation Print
Print






