Abstract
Background/Aims
In Korea, increasing clarithromycin resistance has led to the need for an alternative first-line therapy for the eradication of Helicobacter pylori (H. pylori) infection. Concomitant therapy (CT) and sequential therapy (ST) have been proposed as alternative regimens. The aim of this study was to compare the eradication rate from using CT and ST in Korea.
Methods
A literature review was performed on studies comparing the efficacy of CT and ST in Korea. Data were pooled to obtain the odds ratio (OR) of the eradication rate with 95% confidence intervals (CIs). The eradication rates were considered both on an intention-to-treat (ITT) and a per-protocol (PP) bases.
Guidelines for the diagnosis and treatment of Helicobacter pylori (H. pylori) infection were first developed in 1998 by the Korean H. pylori study group, and updated in 2009 and 2013.12 Most international guidelines have recommended a regimen of triple therapy (TT) consisting of proton pump inhibitor (PPI), amoxicillin or metronidazole, and clarithromycin as the first line therapy to eradicate H. pylori. However, the eradication rate from using TT has been declining in the past decades worldwide, and is now considered to be below 80% in Korea.34 Nevertheless, according to the 2013 revised Korean guideline, TT is still recommended as the first line therapy since there is no better option to date. Thus, it is urgent to develop a more effective strategy. Concomitant therapy (CT) and sequential therapy (ST) have been proposed as alternatives to TT. They have been studied vigorously in various countries including Korea. Most of these studies concluded that CT showed better outcome compared with ST. However, previous meta-analyses failed to show the superiority of CT compared with ST.5
We assumed that there might be a regional difference in the eradication rate of each regimen and performed a systematic review and meta-analysis of the studies comparing CT and ST in Korea.
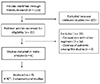
According to the preferred reporting items for Systemic Reviews and Meta-Analyses (PRISMA) protocols, we searched PubMed, EMBASE, KoreaMed, and Cochrane Library for studies comparing CT with ST for H. pylori infection.6 Combinations of the following key words were used in the search: “Helicobacter pylori”, “concomitant therapy”, “sequential therapy”, “disease eradication”, “therapeutics”, and “drug therapy”. The results of the search were reviewed by two authors (HJ Bae and JS Kim).
Studies were eligible if they included both CT and ST as the first-line therapy, and met the following criteria: (1) diagnosis of H. pylori infection by at least one of the following methods: rapid urease test, urea breath test, histology, or culture; (2) confirmation of eradication of infection by an appropriate follow-up test; (3) CT consisting of a PPI, amoxicillin, clarithromycin, and metronidazole given concomitantly for 7-14 days; and (4) ST consisting of PPI and amoxicillin for the first 5-7 days, followed by triple therapy, including PPI, clarithromycin, and metronidazole for the remaining 5-7 days. Case reports, letters, editorials, commentaries, reviews, and abstracts were excluded.
A manual for data extraction was made ahead of time, and two authors (HJ Bae and JS Kim) collected the data independently. The following items were extracted from the selected articles: study design; number of patients enrolled each study in total and in respective treatment groups; diagnostic methods of testing H. pylori infection before enrollment and after the end of treatment; drug regimens; number of patients who were successfully eradicated for corresponding regimens; and the incidence of adverse events. The extracted data were sorted out independently by the two authors, and disagreements were resolved by consensus. We tried to contact the original authors if we need additional information.
We used two different risk assessment tools according to the study designs. The risk of bias for randomized controlled trials (RCTs) was assessed using the tool developed by Cochrane collaboration.7 The criteria were randomization (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Each part was recorded as yes (Y), no (N), or unclear (U), and any disagreements were resolved by consensus. The risk of bias for non-randomized studies was assessed by Newcastle-Ottawa scale.8 It was categorized to three parts: selection (composed of four individual questions), comparability of study groups (of two questions), and ascertainment of exposure or outcome (of three questions). Each question was scored for one point, and the studies receiving more than six points were considered as high quality.
The pooled odds ratio (OR) with 95% confidence intervals (CIs) were calculated. The eradication rates were analyzed on both intention-to-treat (ITT) population and per-protocol (PP) population. Full application of ITT analysis can only be performed for RCTs; thus, we excluded retrospective studies for ITT analysis. We evaluated the heterogeneity between the pooled estimates using Cochran's Q test and I2 statistics. When there was high heterogeneity (p<0.1 or I2>50), the random-effect model should be used to combine the effect sizes of the included studies. However, a nonsignificant test only represents a scarcity of evidence for heterogeneity, as there may have been insufficient power to detect any heterogeneity. Therefore, we decided to take the pooled OR on a conservative approach and used a random-effects model. Subgroup analysis and meta-regression were also performed when needed. To evaluate the discrepancies of small scale studies and publication bias, we also conducted Egger's test and formed a funnel plot when necessary. Statistical analyses were executed by the aid of Comprehensive meta-analysis software version 2 (Biostat Inc., Englewood, NJ, USA).
The main characteristics of the studies are presented in Tables 1 and 2. Four studies were single-centered, whereas the remaining two studies were multi-centered design. The enrollment period ranged between 2010 and 2015. Two studies used pantoprazole, three studies used rabeprazole, and the remaining one study used lansoprazole for CT and ST regimens. Two studies used metronidazole 500 mg three times a day, while the rest used metronidazole two times a day. Treatment durations were 7, 10, and 14 days for CT, and 10 and 14 days for ST.
A total of 1897 Korean patients were included; of whom 917 were treated with CT and 980 were treated with ST. The pooled ITT eradication rates were 82.53% (95% CI: 71.0-94.1) for CT and 77.54% (95% CI: 64.8-90.2) for ST. The pooled PP eradication rates were 85.50% (95% CI: 78.6-92.4) for ST and 91.50% (95% CI: 83.8-99.2) for CT. The pooled OR of ITT eradication rates was 1.382 (95% CI: 1.031-1.853, p=0.031) (Fig. 2). The pooled OR of PP eradication rates was 2.114 (95% CI: 1.502-2.974, p<0.001) (Fig. 3). The pooled OR of PP eradication rates for RCTs was 2.102 (95% CI: 1.346-3.282, p=0.001).
There was some funnel plot asymmetry (Fig. 4), which was compatible with publication bias; however, statistical analysis using Egger's test showed no significance (p=0.426). These results should be interpreted with caution because the asymmetry test is probably underpowered as there were only six studies included in this meta-analysis.
No significant heterogeneity was observed among the studies for adverse events (p=0.1401, I2=42.21%). The pooled OR for adverse events was 1.171 (95% CI: 0.883-1.554, p=0.274) and the corresponding rates were 39.50% (95% CI: 30.3-48.7) for CT and 37.79% (95% CI: 31.7-43.9) for ST (Fig. 5). Low heterogeneity was observed among the studies for compliance to the medications (p=0.7056, I2=0.0%). The pooled OR for compliance was 0.734 (95% CI: 0.407-1.322, p=0.303) and the corresponding rates were 96.78% (95% CI: 95.1-98.4) for CT and 96.95% (95% CI: 94.0-99.9) for ST.
Except for one study, all other studies included a sequential therapy of 10 days. However, the duration of CT varied, from 7, to 14 days. Hence, we performed a subgroup analysis based on the duration of CT. Three studies compared 10 days of concomitant therapy with 10 days of sequential therapy. The pooled OR of eradication rates were 2.617 (95% CI: 1.484-4.615, p=0.001) favoring CT. Two studies compared 7 days of concomitant therapy with 10 days of ST. The pooled OR of eradication rates were 2.079 (95% CI: 1.073-4.029, p=0.030) favoring CT. We did not perform a subgroup analysis if the duration of CT was longer than the duration of ST. Moreover, because all but one study tested a sequential therapy lasting 10 days, a subgroup analysis according to the duration of ST was not performed.
The eradication rate of TT for H. pylori has been declining recently in Korea.4 The main cause of this tendency is likely due to increased antibiotic resistance of H. pylori.15 Many Korean gastroenterologists are struggling to find a regimen that can overcome this issue. CT and ST have been proposed as alternative regimens by various countries161718 and studied vigorously for the past several years in Korea.161719
Previous meta-analyses have compared the eradication rates of ST and CT. One meta-analysis reported that CT did not achieve a higher eradication rate compared with ST.5 However, a recent meta-analysis, including 20 randomized controlled trials, reported that 10 days of CT regimen appears to be superior to a 10 days of ST regimen.18 Korea is a region with high antibiotic resistance to clarithromycin and metronidazole. Therefore, we decided to perform a meta-analysis regarding studies performed only in Korea.
This meta-analysis showed that CT was superior compared with ST with respect to the eradication of H. pylori in Korea. There was no difference in adverse events and compliance between the two regimens. To the best of our knowledge, this is the first meta-analysis comparing CT and ST as first-line therapy based only on the Korean population. Considering that the eradication rate of TT is rapidly declining in Korea, CT might replace TT as the first line therapy.
However, we could not conclude the optimal duration of CT through our analysis. Subgroup analysis showed both 7-day and 10-day regimen of CT to be better than10-day of ST; however, direct comparison between 7 and 10 days of CT has not been performed due to limited data.
The results of our study correlates to previous studies and further shows that ST is inferior to CT. High metronidazole resistance rates may be the reason for these results. It is known that metronidazole resistance can be partially overcome by increasing the dose, frequency, and duration of antibiotics.16 While a regimen of ST provides metronidazole for 5-7 days, a regimen of CT provides metronidazole for 10-14 days. The longer duration of metronidazole use might overcome resistance, resulting in increased eradication rates for the CT regimen.
There are some limitations to consider when interpreting our findings. First, the studies included in this analysis were small, and there were two retrospective studies included. Hence, we could not precisely measure the publications bias and examine correlation between the duration of CT and eradication rates. Second, there was no single study that reported antibiotics resistance and its association with the eradication rates. At first, CT and ST were developed due to increasing antibiotics resistance. However, since antibiotic resistance was not available in any study, we were unable to compare which regimen was more superior to antibiotic-resistant strains.
In conclusion, for the Korean population, it seems, with statistical significance, that CT is more effective than ST as the first line therapy to eradicate H. pylori infection. There were no differences in adverse events and compliance between the two regimens. Further prospective studies regarding the optimal duration of CT, with an investigation of its effectiveness on antibiotic-resistant strains are necessary.
Figures and Tables
 | Fig. 2Forest plot of intention-to-treat analysis of CT compared with ST. CI, confidence interval; ST, sequential therapy; CT, concomitant therapy. |
 | Fig. 3Forest plot of per-protocol analysis of CT compared with ST. CI, confidence interval; ST, sequential therapy; CT, concomitant therapy. |
 | Fig. 4Funnel plot asymmetry test for the detection of bias within the studies for the eradication rate of concomitant therapy compared with sequential therapy. |
 | Fig. 5Forest plot of adverse events of concomitant therapy CT compared with sequential therapy ST. CI, confidence interval; ST, sequential therapy; CT, concomitant therapy. |
References
1. Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009; 54:269–278.

2. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.

3. Papastergiou V, Georgopoulos SD, Karatapanis S. Current and future insights in H. pylori eradication regimens: the need of tailoring therapy. Curr Pharm Des. 2014; 20:4521–4532.

4. Kim YK, Kim JS, Kim BW. Recent trends of Helicobacter pylori eradication therapy in Korea. Korean J Helicobacter Up Gastrointest Res. 2012; 12:219–223.
5. Kim JS, Park SM, Kim BW. Sequential or concomitant therapy for eradication of Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2015; 30:1338–1345.
6. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–e34.

7. Higgins JPT, Altman DG, Sterne JAC. Cochrnae handbook for systematic reviews of interventions, version 5.1.0. Chapter 8: assessing risk of bias in included studies. [Internet]. London: Cochrane Training;2006. 09. updated Mar 2011. cited 2016 Jan 31. Available from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
8. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605.

9. Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2017; 32:589–594.
10. Chung JW, Han JP, Kim KO, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: a multicenter, prospective study. Dig Liver Dis. 2016; 48:888–892.

11. Lee HJ, Kim JI, Lee JS, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015; 21:351–359.
12. Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013; 18:180–186.
13. Jung SM, Cheung DY, Kim JI, Kim I, Seong H. Comparing the efficacy of concomitant therapy with sequential therapy as the first-line therapy of Helicobacter pylori eradication. Gastroenterol Res Pract. 2016; 2016:1293649.
14. Kim SY, Park DK, Kwon KA, Kim KO, Kim YJ, Chung JW. Ten day concomitant therapy is superior to ten day sequential therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2014; 64:260–267.
15. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
16. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017; 112:212–239.

17. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.
18. Wang Y, Zhao R, Wang B, et al. Sequential versus concomitant therapy for treatment of Helicobacter pylori infection: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2018; 74:1–13.

19. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30.




 ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download