Abstract
Background/Aims
Moxifloxacin-based sequential therapy showed an excellent eradication rate as the first line treatment of Helicobacter pylori (H. pylori) infection. However, to the best of our knowledge, there were only a few studies on the treatment of those with failed moxifloxacin-based sequential therapy. Hence, this study was to investigate the efficacy of bismuth-containing quadruple therapy in those with failed moxifloxacin-based sequential or reverse sequential therapy for H. pylori eradication.
Methods
Between January 2013 and March 2016, we retrospectively analyzed patients who failed to eradicate H. pylori using moxi-floxacin-based sequential (rabeprazole 20 mg bid and amoxicillin 1 g bid for 5–7 days, followed by rabeprazole 20 mg bid, metronidazole 500 mg bid, and moxifloxacin 400 mg qd for 5–7 days) and 10 days moxifloxacin-based reverse sequential therapy as the first line treatment. Then we investigated the eradication rates of bismuth-containing quadruple therapy as the second line treatment. All subjects had no history of H. pylori eradication before. Eradication rates were described as intention-to-treat (ITT) and per-protocol (PP) analyses. H. pylori status was evaluated by 13 C-urea breath test 6 weeks after the end of the treatment. Moreover, we examined any side effects that caused discontinuation of therapy.
Go to : 
References
1. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.

2. Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004; 10:668–671.
3. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:321–331.

4. De Francesco V, Margiotta M, Zullo A, et al. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. J Antimicrob Chemother. 2007; 59:783–785.

5. Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and metaanalysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009; 104:3069–3079. quiz 1080.
6. Yoon H, Lee DH, Kim N, et al. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013; 28:1801–1809.

7. Park HG, Jung MK, Jung JT, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naïve patients. Aliment Pharmacol Ther. 2012; 35:56–65.
8. Kwon JH, Lee DH, Song BJ, et al. Ten-day sequential therapy as first-line treatment for Helicobacter pylori infection in Korea: a retrospective study. Helicobacter. 2010; 15:148–153.
9. Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. Efficacy of moxifloxacin-based sequential and hybrid therapy for first-line Helicobacter pylori eradication. World J Gastroenterol. 2015; 21:10234–10241.
10. Tsay FW, Wu DC, Kao SS, et al. Reverse sequential therapy achieves a similar eradication rate as standard sequential therapy for Helicobacter pylori eradication: a randomized controlled trial. Helicobacter. 2015; 20:71–77.
11. Shaikh T, Fallone CA. Effectiveness of second through sixth line salvage Helicobacter pylori treatment: bismuth quadruple therapy is almost always a reasonable choice. Can J Gastroenterol Hepatol. 2016; 2016:7321574.
12. Chung JW, Lee JH, Jung HY, et al. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter. 2011; 16:289–294.

13. Lee BH, Kim N, Hwang TJ, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010; 15:38–45.
14. Cheon JH, Kim N, Lee DH, et al. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006; 11:46–51.

15. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and anti-biotic resistance. Dig Dis Sci. 2014; 59:1235–1243.

16. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011; 17:3971–3975.

17. Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2001; 15:1023–1029.

18. Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, ni-tazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011; 106:1970–1975.

19. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.

20. Nishizawa T, Suzuki H, Suzuki M, Takahashi M, Hibi T. Proton pump inhibitor-amoxicillin-clarithromycin versus proton pump inhibitor-amoxicillin-metronidazole as first-line Helicobacter pylori eradication therapy. J Clin Biochem Nutr. 2012; 51:114–116.

21. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.
22. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
23. Gong EJ, Yun SC, Jung HY, et al. Meta-analysis of first-line triple therapy for helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 2014; 29:704–713.

24. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.

25. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14:109–118.
26. Kim JS, Kim BW, Ham JH, et al. Sequential therapy for Helicobacter pylori infection in Korea: systematic review and metaanalysis. Gut Liver. 2013; 7:546–551.

27. Keating GM, Scott LJ. Moxifloxacin: a review of its use in the management of bacterial infections. Drugs. 2004; 64:2347–2377.
28. Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis. 2000; 32:81–85.
29. Kale-Pradhan PB, Mihaescu A, Wilhelm SM. Fluoroquinolone sequential therapy for Helicobacter pylori: a metaanalysis. Pharmacotherapy. 2015; 35:719–730.

30. Hwang JJ, Lee DH, Lee AR, et al. Efficacy of moxifloxacin-based sequential therapy for first-line eradication of Helicobacter pylori infection in gastrointestinal disease. World J Gastroenterol. 2015; 21:5032–5038.
31. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
32. Gisbert JP, Molina-Infante J, Marin AC, Vinagre G, Barrio J, McNicholl AG. Second-line rescue triple therapy with levofloxacin after failure of non-bismuth quadruple "sequential" or "concomitant" treatment to eradicate H. pylori infection. Scand J Gastroenterol. 2013; 48:652–656.
33. Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacinbased triple therapy for Helicobacter pylori retreatment: role of bacterial resistance. Dig Liver Dis. 2007; 39:1001–1005.

34. Manfredi M, Bizzarri B, de'Angelis GL. Helicobacter pylori infection: sequential therapy followed by levofloxacin-containing triple therapy provides a good cumulative eradication rate. Helicobacter. 2012; 17:246–253.

35. Pontone S, Standoli M, Angelini R, Pontone P. Efficacy of H. pylori eradication with a sequential regimen followed by rescue therapy in clinical practice. Dig Liver Dis. 2010; 42:541–543.

36. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006; 28:6–13.

37. Hwang JJ, Lee DH, Lee AR, et al. Fourteen- vs seven-day bismuth-based quadruple therapy for second-line Helicobacter pylori eradication. World J Gastroenterol. 2015; 21:8132–8139.
38. Muller N, Amiot A, Le Thuaut A, Bastuji-Garin S, Deforges L, Delchier JC. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol. 2016; 40:517–524.

39. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017; 112:212–239.

40. Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013; 88:33–45.
Go to : 
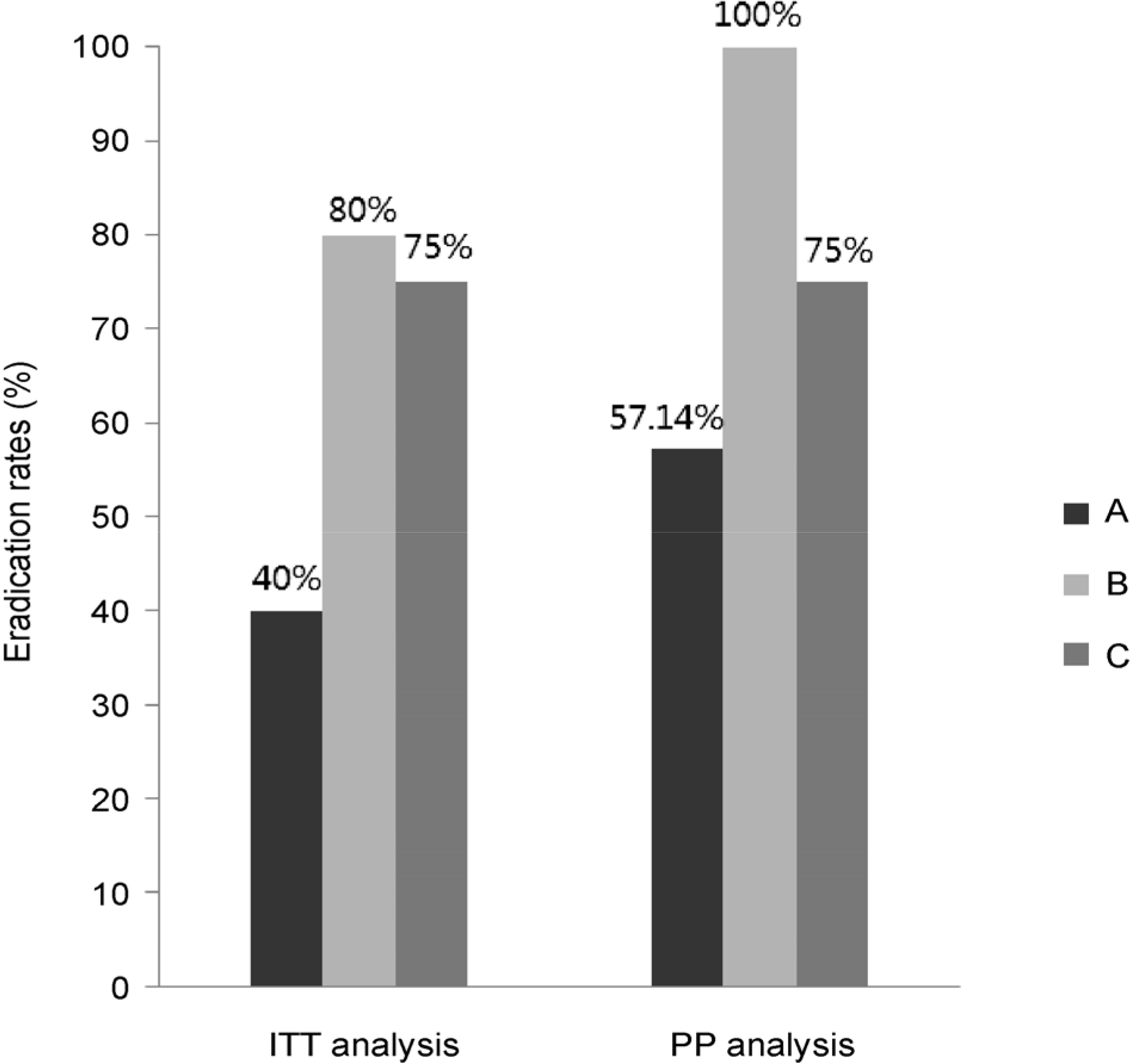 | Fig. 1.Comparison of eradication rates according to the first line therapy. Eradication rates are presented by intention-to-treat (ITT) and per-protocol (PP) analyses. There was no statistically significant difference according to the first line therapy by both ITT (p-value= 0.257) and PP analyses (p-value=0.278). A, 10 days sequential; B, 14 days sequential; C, reverse sequential. |
Table 1.
Baseline Characteristics of Enrolled Patients according to the First Line Therapy
| Variable | 10 days sequential (n=10) | 14 days sequential (n=5) | Reverse sequential (n=8) | p-value |
|---|---|---|---|---|
| Age | 63.50±1.59 | 59.4±3.36 | 61.0±2.95 | 0.125 |
| Sex | ||||
| Male | 6 (60) | 3 (60) | 2 (25) | 0.294 |
| Female | 4 (40) | 2 (40) | 6 (75) | 0.294 |
| Current smoker | 0 | 0 | 0 | |
| Alcohol consumption | 0 | 0 | 0 | |
| EGD findings | 0.052 | |||
| Gastritis | 9 (90) | 5 (100) | 7 (87.5) | |
| Duodenal ulcer | 1 (10) | 0 | 1 (12.5) | |
| Underlying diseases | ||||
| HTN | 4 (50) | 1 (12.5) | 3 (37.5) | 0.743 |
| DM | 1 (100) | 0 | 0 | >0.999 |
| Hyperlipidemia | 1 (50) | 0 | 1 (50) | >0.999 |
| Thyroid disease | 0 | 1 (50) | 1 (50) | 0.308 |
| Time interval (days) a | 67.42±43.56 | 63.86±36.23 | 77.40±64.07 | 0.125 |
| Second line treatment results | ||||
| Eradicated | 4 (40) | 4 (80) | 6 (75) | 0.257 |
| Not eradicated | 3 (30) | 0 | 2 (25) | 0.563 |
| Follow up loss | 3 (30) | 1 (20) | 0 | 0.298 |
Table 2.
Comparison of Eradicated and not Eradicated Groups
| Variable | Total (n=19) | Eradicated (n=14) | Not eradicated (n=5) | p-value |
|---|---|---|---|---|
| Age | 62.74±6.38 | 62.50±7.08 | 63.40±4.39 | 0.964 |
| Sex | ||||
| Male | 9 (47.4) | 7 (50) | 2 (40) | >0.99 |
| Female | 10 (52.6) | 7 (50) | 3 (60) | >0.99 |
| Current smoker | 0 | 0 | 0 | |
| Alcohol consumption | 0 | 0 | 0 | |
| EGD findings | 0.052 | |||
| Gastritis | 18 (94.7) | 18 (100) | 0 | |
| Duodenal ulcer | 1 (5.3) | 0 | 1 (100) | |
| Underlying diseases | ||||
| HTN | 7 (100) | 6 (85.7) | 1 (14.3) | 0.602 |
| Hyperlipidemia | 2 (100) | 1 (50) | 1 (50) | >0.999 |
| Thyroid disease | 1 (100) | 1 (100) | 0 | >0.999 |
| Time intervals (days)a | 67.42±43.56 | 63.86±36.23 | 77.40±64.07 | 0.964 |
| First line therapy regimen | 0.278 | |||
| 10 days sequential | 7 (36.8) | 4 (57.14) | 3 (42.86) | |
| 14 days sequential | 4 (21.1) | 4 (100) | 0 | |
| Reverse sequential | 8 (42.1) | 6 (75) | 2 (25) | |
| Adverse events b | ||||
| Nausea | 4 (21.1) | 3 (75) | 1 (25) | >0.999 |
| Epigastric soreness | 2 (10.5) | 2 (100) | 0 | 0.687 |
| Dyspepsia | 1 (5.3) | 1 (100) | 0 | 0.823 |
| Abdominal discomfort | 1 (5.3) | 0 | 1 (100) | 0.559 |
| Fatigue | 1 (5.3) | 0 | 1 (100) | 0.559 |
| Diarrhea | 1 (5.3) | 1 (100) | 0 | 0.823 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download