Abstract
When liver disease is severe, the prognosis can be worse if the patient is malnourished. Adequate nutritional support for patients with liver diseases can improve the patient's condition and prognosis. In the case of liver cirrhosis, malnutrition can occur due to a variety of causes, including poor oral intake, maldigestion, malabsorption, associated renal disease, and metabolic abnormalities. For a nutritional assessment, it is important to check the dietary intake, change in body composition, including anthropometry, and a functional assessment of muscle. Counselling and oral or enteral nutrition is preferred over parenteral nutrition as in other diseases. If esophageal varices are present, care should be taken when installing a feeding tube, but if there are ascites, percutaneous endoscopic gastrostomy is contraindicated because of the risk of complications. Calories of 30–35 kcal/kg/day and protein from 1.2 to 1.5 g/kg/day are appropriate. Protein restriction is unnecessary unless the hepatic encephalopathy is severe. A late evening snack and branched chain amino acids can be helpful. In the case of cholestasis, the supply of manganese and copper should be restricted. Sarcopenia in patients with liver cirrhosis is also prevalent and associated with the prognosis.
Go to : 
References
1. Child CG, Turcotte JG. Surgery and portal hypertension. Child CG, editor. The liver and portal hypertension. Philadelphia: Saunders;1964. p. 50–51.
2. Guglielmi FW, Panella C, Buda A, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005; 37:681–688.
3. Müller MJ, Böttcher J, Selberg O, et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999; 69:1194–1201.

4. Nutritional status in cirrhosis. Italian multicentre cooperative project on nutrition in liver cirrhosis. J Hepatol. 1994; 21:317–325.
5. Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014; 18:179–190.

6. Charles M, Sarah M, Denise S, Deb K, Steve M. The A.S.P.E.N. Adult Nutrition Support Core Curriculum. 2nd ed.Silver Spring (MD): American Society for Parenteral & Enteral Nutrition;2012.
7. Mouzaki M, Ng V, Kamath BM, Selzner N, Pencharz P, Ling SC. Enteral energy and macronutrients in end-stage liver disease. JPEN J Parenter Enteral Nutr. 2014; 38:673–681.

8. Koretz RL. The evidence for the use of nutritional support in liver disease. Curr Opin Gastroenterol. 2014; 30:208–214.

9. Arakawa Y, Moriyama M, Arakawa Y. Liver cirrhosis and metabolism (sugar, protein, fat and trace elements). Hepatol Res. 2004; 30S:46–58.

10. Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017; 65:1044–1057.

11. Arora S, Mattina C, McAnenny C, et al. The development and validation of a nutritional prioritising tool for use in patients with chronic liver disease. J Hepatol. 2012; 56(Suppl2):S241.
12. Borhofen SM, Gerner C, Lehmann J, et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016; 61:1735–1743.

13. Booi AN, Menendez J, Norton HJ, Anderson WE, Ellis AC. Validation of a screening tool to identify undernutrition in ambulatory patients with liver cirrhosis. Nutr Clin Pract. 2015; 30:683–689.

14. Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017; 36:49–64.

15. Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013; 28:15–29.

16. Alvares-da-Silva MR, da Reverbel ST. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005; 21:113–117.

17. Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016; 111:1759–1767.

18. Ney M, Haykowsky MJ, Vandermeer B, Shah A, Ow M, Tandon P. Systematic review: pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment Pharmacol Ther. 2016; 44:796–806.

19. Tandon P, Low G, Mourtzakis M, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016; 14:1473–1480.

20. Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005; 21:113–117.

21. Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006; 44:823–835.

22. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). JPEN J Parenter Enteral Nutr. 2016; 40:159–211.
24. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997; 16:43–55.

25. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N). JPEN J Parenter Enteral Nutr. 2009; 33:277–316.
26. Ministry of Health and Welfare. Ministry of Health and Welfare Notice No. 2016–259. [Internet]. Sejong (KR): Ministry of Health and Welfare;2016 Dec 28. [updated 2017 Jan 16;cited 2018 Mar 14]. Available from:. http://www.mohw.go.kr/react/jb/sjb0406ls.jsp?PAR_MENU_ID=03&MENU_ID=030406&page=28#.
27. Lo GH, Lin CW, Hsu YC. A controlled trial of early versus delayed feeding following ligation in the control of acute esophageal variceal bleeding. J Chin Med Assoc. 2015; 78:642–647.

28. de Lédinghen V, Beau P, Mannant PR, et al. Early feeding or enteral nutrition in patients with cirrhosis after bleeding from esophageal varices? A randomized controlled study. Dig Dis Sci. 1997; 42:536–541.
29. Andus T. ESPEN guidelines on enteral nutrition: liver disease – tube feeding (TF) in patients with esophageal varices is not proven to be safe. Clin Nutr. 2007; 26:272. author reply 273–274.
30. Plauth M, Cabré E, Riggio O, et al. ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr. 2006; 25:285–294.

31. Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: ex-ploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012; 27:430–441.

32. ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002; 26(1 Suppl):1SA–138SA.
33. Kawaguchi T, Izumi N, Charlton MR, Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011; 54:1063–1070.

34. Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003; 124:1792–1801.

35. Muto Y, Sato S, Watanabe A, et al. Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005; 3:705–713.
36. Gluud LL, Dam G, Borre M, et al. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr. 2013; 143:1263–1268.

37. Metcalfe EL, Avenell A, Fraser A. Branched-chain amino acid supplementation in adults with cirrhosis and portosystemic encephalopathy: systematic review. Clin Nutr. 2014; 33:958–965.

38. Plauth M, Cabré E, Campillo B, et al. ESPEN guidelines on parenteral nutrition: hepatology. Clin Nutr. 2009; 28:436–444.

40. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010; 39:412–423.

41. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and metaanalysis. PLoS One. 2017; 12:e0186990.

42. Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015; 6:e102.

43. van Vugt JLA, Alferink LJM, Buettner S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018; 68:707–714.

44. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis–aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016; 43:765–777.
45. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998; 85:115–122.
Go to : 
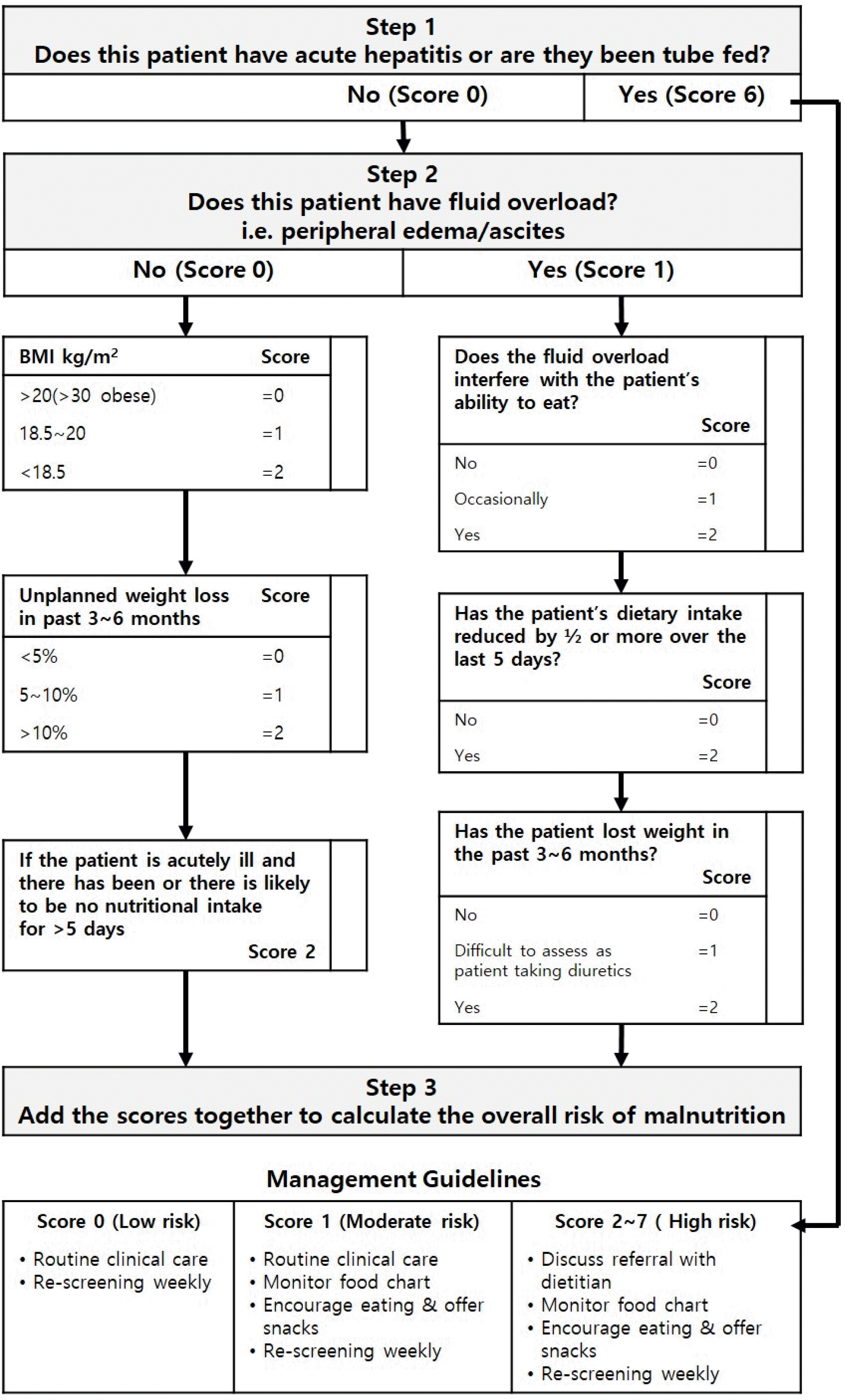 | Fig. 1.Royal Free Hospital-Nutritional Prioritizing Tool. Adapted from Arora et al.11, with permission from J Hepatol. |
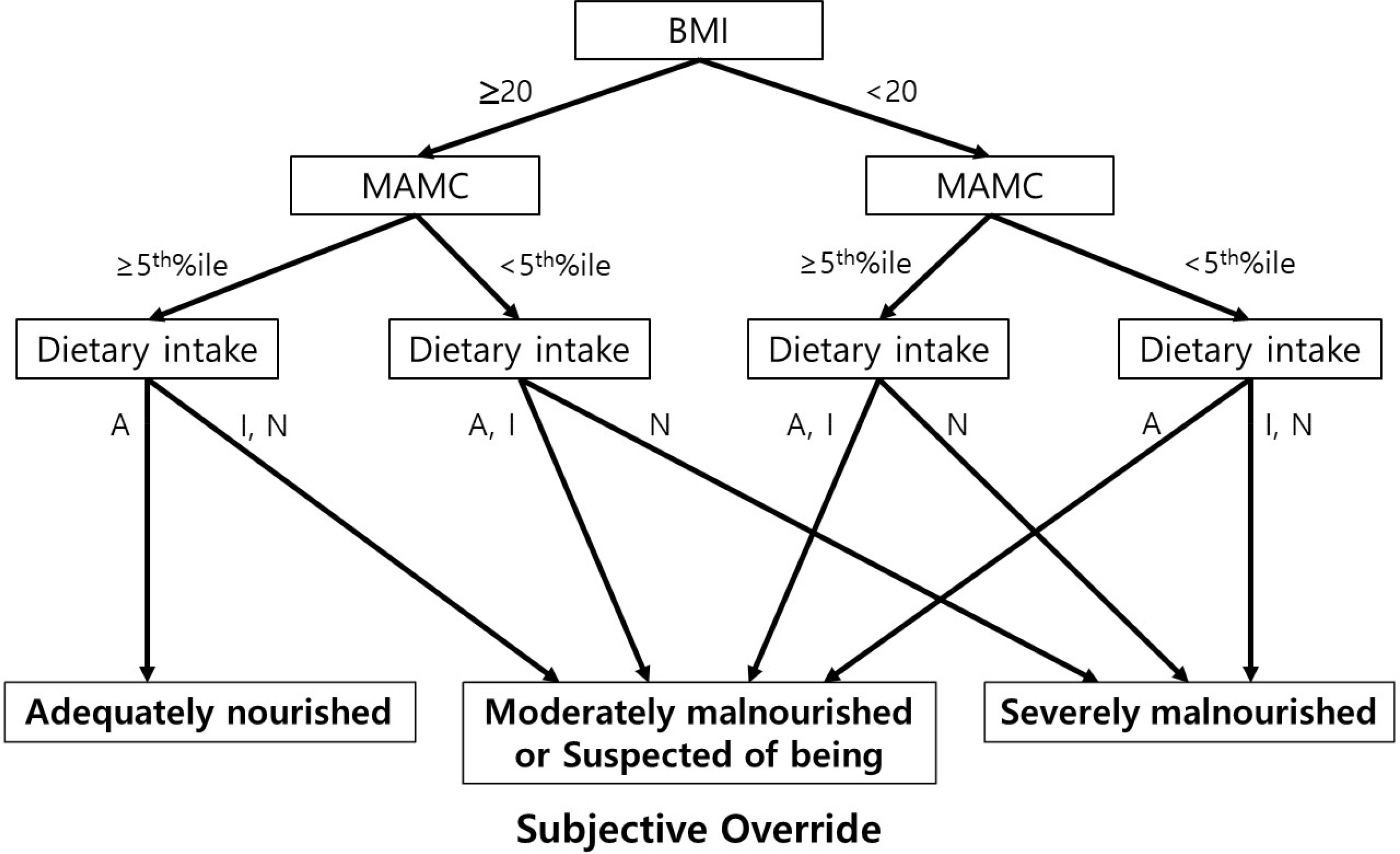 | Fig. 2.Royal Free Hospital Global Assessment scheme. BMI, body mass index; MAMC, mid- arm muscle cir-cumference; A, adequate; I, inadequate; N, negligible. Adapted from Morgan et al.21, with permission from Hepatology. |
Table 1.
Summary of Nutrition Screening Tools (Adapted from Tandon et al.10, with permission from Hepatology)
MUST, malnutrition universal screening tool; NRS-2002, nutritional risk screening 2002; NUTRIC, nutrition risk in critically Ill; MNA, mini nutritional assessment; SNAQ, short nutritional assessment questionnaire; MST, malnutrition screening tool; RFH-NPT, royal free hospital-nutritional prioritizing tool; CNST, Canadian nutrition screening tool; BMI, body mass index; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; ICU, intensive care unit; GI, gastrointestinal.
Table 2.
Tools for Assessing the Oral Protein-energy Intake in End Stage Liver Disease (Adapted from Johnson et al.15, with permission from Nutr Clin Pract)
Table 3.
Summary of Nutritional Management in Patients with End Stage Liver Disease




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download