Abstract
Background/Aims
Dysphagia is encountered in a large proportion of patients with lung cancer and is associated with malnutrition and a poor quality of life. This study compared the clinical outcomes of self-expandable metallic stent (SEMS) insertion and percutaneous gastrostomy (PG) feeding for patients with lung cancer and dysphagia.
Methods
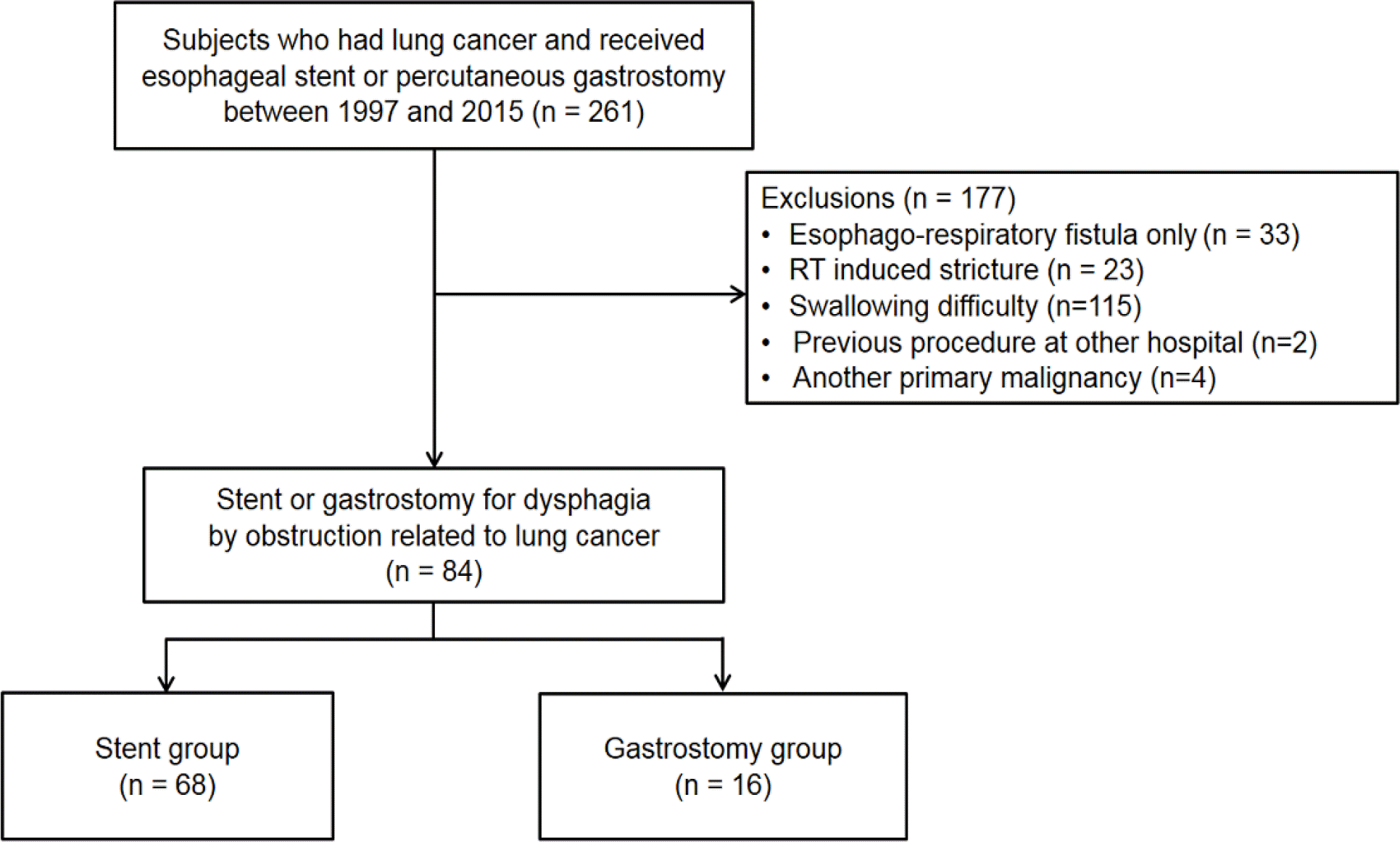
A total of 261 patients with lung cancer, who underwent either SEMS insertion (stent group) or PG (gastrostomy group) as an initial treatment procedure for dysphagia between July 1997 and July 2015 at the Samsung Medical Center, were reviewed retrospectively, and 84 patients with esophageal obstruction were identified. The clinical outcomes, including the overall survival, additional intervention, complications, and post-procedural nutritional status in the two groups, were compared.
Results
Among the 84 patients finally analyzed, 68 patients received SEMS insertion and 16 had PG. The stent group had less cervical obstruction and more mid-esophageal obstruction than the gastrostomy group. The Kaplan-Meier curves revealed similar overall survival in the two groups. Multivariate analysis showed that the two modalities had similar survival rates (PG compared with SEMS insertion, hazard ratio 0.682, p=0.219). Fifteen patients (22.1%) in the stent group received additional intervention, whereas there was no case in the gastrostomy group (p=0.063). The decrease in the serum albumin level after the procedure was lower in the gastrostomy group than in the stent group (−0.20±0.54 g/dL vs. −0.65±0.57 g/dL, p=0.013)
Go to : 
References
1. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and dis-ability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017; 3:524–548.
4. Maguire PD, Sibley GS, Zhou SM, et al. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999; 45:97–103.

6. Altemur Karamustafaoglu Y, Yoruk Y. Self-expandable abdominal stents placement for the palliation of dysphagia as a result of lung cancer. Dis Esophagus. 2010; 23:561–564.
7. Tanuma A. Swallowing and voice disorders in cancer patients. Gan To Kagaku Ryoho. 2015; 42:783–786.
8. Writing Group Members. Mozaffarian D, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016; 133:447–454.
9. Christie NA, Buenaventura PO, Fernando HC, et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: short-term and long-term follow-up. Ann Thorac Surg. 2001; 71:1797–1801. discussion 1801–1802.

10. Grilo A, Santos CA, Fonseca J. Percutaneous endoscopic abdominal for nutritional palliation of upper esophageal cancer unsuitable for esophageal stenting. Arq Gastroenterol. 2012; 49:227–231.
11. Homs MY, Steyerberg EW, Kuipers EJ, et al. Causes and abdominal of recurrent dysphagia after self-expanding metal stent placement for palliation of esophageal carcinoma. Endoscopy. 2004; 36:880–886.
12. Mangiavillano B, Pagano N, Arena M, et al. Role of stenting in abdominal benign and malignant diseases. World J Gastrointest Endosc. 2015; 7:460–480.
13. Löser C, Aschl G, Hébuterne X, et al. ESPEN guidelines on abdominal enteral nutrition–percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005; 24:848–861.
14. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed.New York: Springer-Verlag;2009.
15. Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One. 2012; 7:e42766.

16. Min YW, Jang EY, Jung JH, et al. Comparison between gastrostomy feeding and self-expandable metal stent insertion for abdominals with esophageal cancer and dysphagia. PLoS One. 2017; 12:e0179522.
17. National Comprehensive Cancer Network. Non-small cell lung cancer (version 8). [Internet]. Washington (PA): National Comprehensive Cancer Network;2017 Jul 24. [updated 2017 Jul 14; cited 2017 Jul 24]. Available from:. http://www.nccn.org/pro-fessionals/physician_gls/pdf/nscl.pdf.
18. National Comprehensive Cancer Network. Small cell lung cancer (version 3). [Internet]. Washington (PA): National Comprehensive Cancer Network;2017 Jul 24. [updated 2017 Feb 23; cited 2017 Jul 24]. Available from:. http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
19. Segura A, Pardo J, Jara C, et al. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin Nutr. 2005; 24:801–814.

20. Mao-de-Ferro S, Serrano M, Ferreira S, et al. Stents in patients with esophageal cancer before chemoradiotherapy: high risk of complications and no impact on the nutritional status. Eur J Clin Nutr. 2016; 70:409–410.

21. Löser C, Wolters S, Fölsch UR. Enteral long-term nutrition via abdominal endoscopic gastrostomy (PEG) in 210 patients: a four-year prospective study. Dig Dis Sci. 1998; 43:2549–2557.
22. Fonseca J, Santos CA, Brito J. Malnutrition and clinical outcome of 234 head and neck cancer patients who underwent perabdominal endoscopic gastrostomy. Nutr Cancer. 2016; 68:589–597.
Go to : 
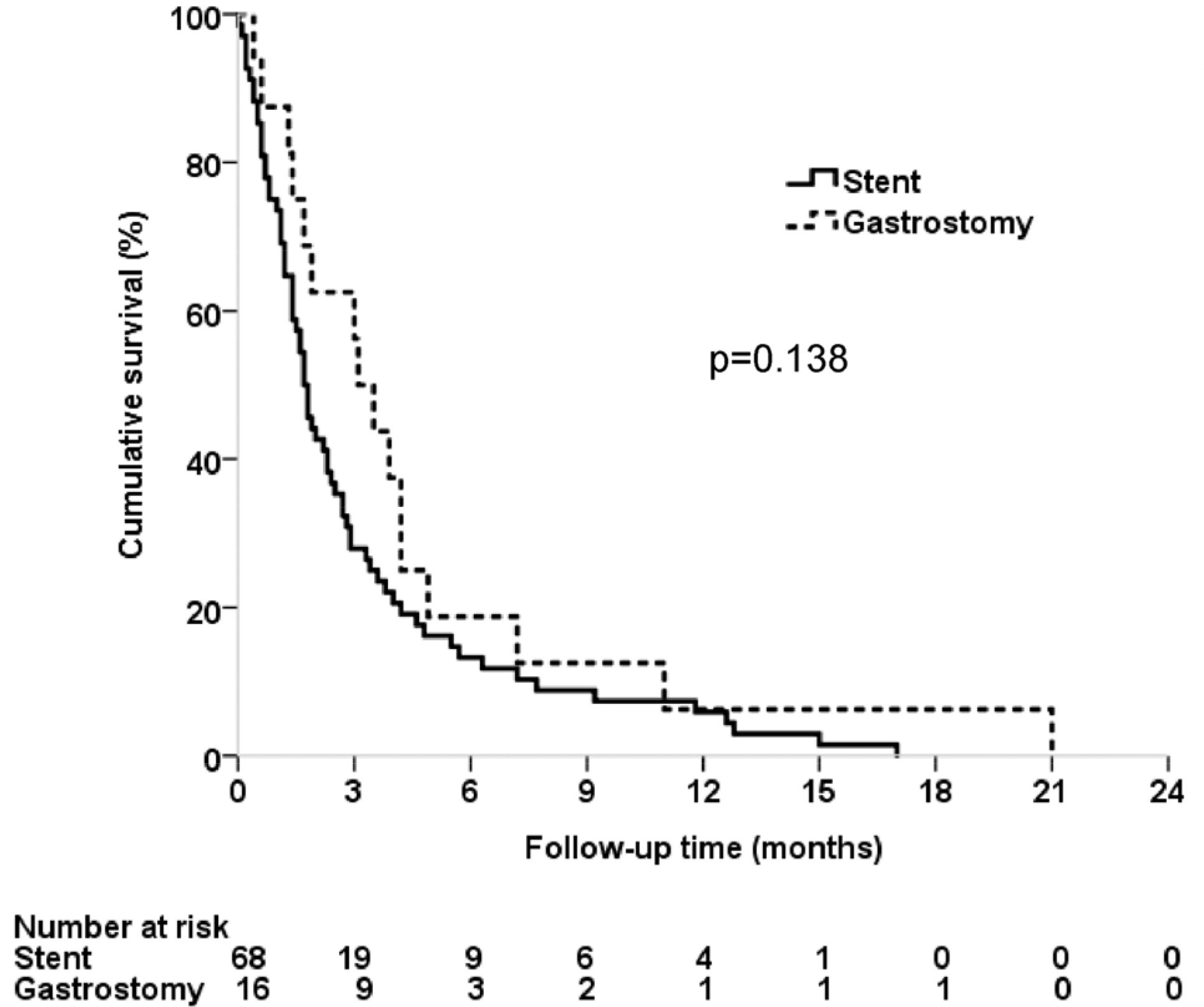 | Fig. 2.Kaplan-Meier curves for the overall survival in lung cancer patients who received either esophageal stent or percutaneous gastrostomy for malignant dysphagia. |
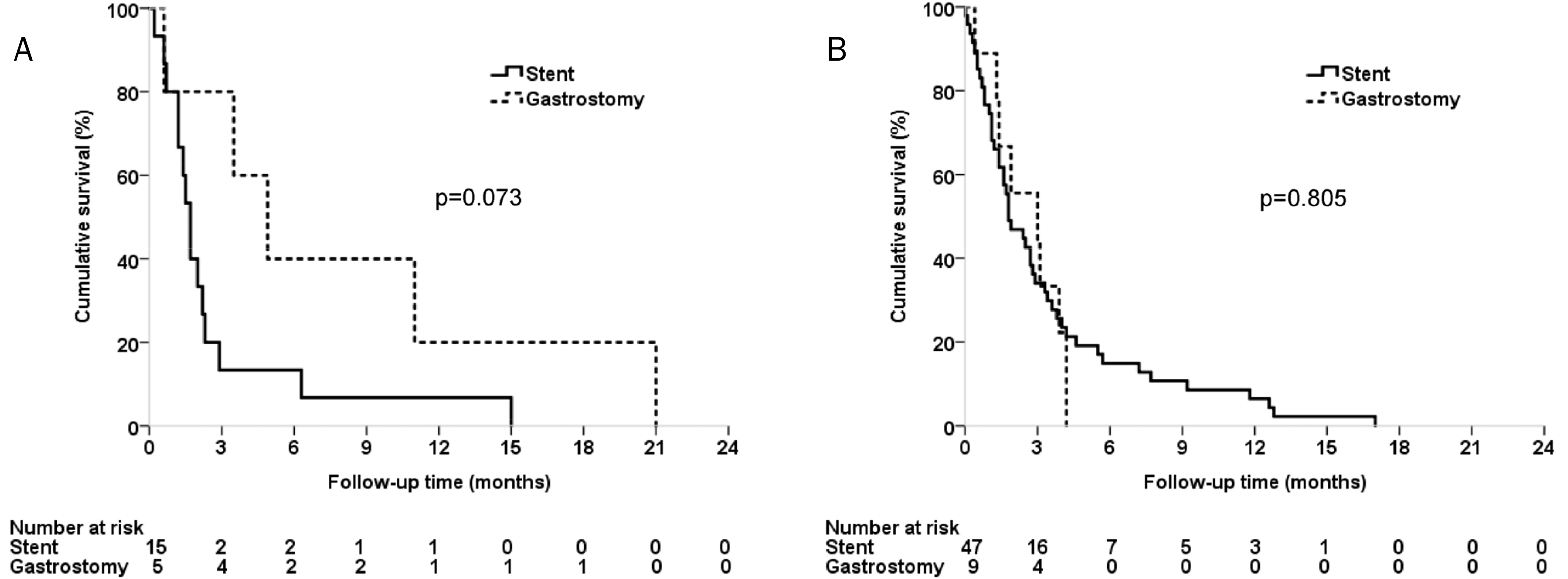 | Fig. 3.Subgroup analysis of the overall survival according to the 7th AJCC staging in NSCLC patients. (A) Kaplan-Meier curves for the overall survival in NSCLC stage III patients who received either esophageal stent or percutaneous gastrostomy for malignant dysphagia. (B) Kaplan-Meier curves for the overall survival in NSCLC stage IV patients who received either esophageal stent or percutaneous gastrostomy for malignant dysphagia. AJCC, American Joint Committee on Cancer; NSCLC, non-small cell lung cancer. |
Table 1.
Comparison of Baseline Characteristics in Lung Cancer Patients Who Received either Esophageal Stent or Percutaneous Gastrostomy for Malignant Dysphagia
| Variables | Stent group (n=68) | Gastrostomy group (n=16) | p-value |
|---|---|---|---|
| Age (years) | 62.4±11.5 | 58.5±6.3 | 0.069 |
| Sex | 0.214 | ||
| Male | 47 (69.1) | 14 (87.5) | |
| Female | 21 (30.9) | 2 (12.5) | |
| BMI (kg/m2) | 20.0±2.68 | 20.2±3.05 | 0.725 |
| Weight (kg) | 51.0 (33.7–78.9) | 54.4 (38.2–70.6) | 0.197 |
| Albumin (g/dL) | 3.63±0.52 | 3.43±0.62 | 0.198 |
| Histology | 0.552 | ||
| Adenocarcinoma | 27 (39.7) | 6 (37.5) | |
| Squamous cell carcinoma | 27 (39.7) | 8 (50.0) | |
| Small cell lung cancer | 6 (8.8) | 2 (12.5) | |
| Others | 8 (11.8) | 0 (0) | |
| Stage by AJCC 7th | 0.611 | ||
| NSCLC stage III | 15 (22.1) | 5 (31.3) | |
| NSCLC stage IV | 47 (69.1) | 9 (56.3) | |
| SCLC a | 6 (8.8) a | 2 (12.5) | |
| Location of stenosis | 0.021 | ||
| Cervical | 1 (1.5) | 2 (15.5) | |
| Upper thoracic | 6 (8.8) | 2 (12.5) | |
| Mid thoracic | 49 (72.1) | 6 (37.5) | |
| Lower thoracic | 12 (17.6) | 6 (37.5) | |
| Stenotic length | 0.530 | ||
| Near total obstruction | 5 (7.4) | 1 (6.3) | |
| ≤3 cm | 24 (35.3) | 5 (31.3) | |
| >3 cm and ≤6 cm | 26 (38.2) | 9 (56.3) | |
| >6 cm | 13 (19.1) | 1 (6.3) | |
| CTx or RTx after procedure | |||
| No | 33 (48.5) | 5 (31.3) | 0.270 |
| Yes | 35 (51.5) | 11 (68.8) | |
| CTx only | 16 | 6 | |
| RTx only | 9 | 3 | |
| CTx and RTx | 10 | 2 |
Table 2.
Prognostic Factors Associated with the Overall Survival in Patients with Dysphagia Induced by a Lung Cancer Obstruction
Table 3.
Comparison of the Secondary Outcomes in Lung Cancer Patients Who Received either Esophageal Stent or Percutaneous Gastrostomy for Malignant Dysphagia




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download