Figures and Tables
Fig. 1
Abdominal computed tomographic findings. (A, B) About 2.7 cm sized intraluminal polypoid mass at the gastric fundus (arrow).

Fig. 2
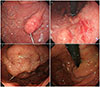
Esophagogastroduodenoscopic findings. (A) About 2.5 cm sized fusiform shaped hyperemic polypoid mass, at the gastric fundus. (B) Focal hyperemic erosion, at the gastric upper body, posterior wall side. (C) Increased size of gastric fundal mass after 1 year (D) Regression of previous mass and erosion after 3rd line chemotherapy.
References
1. Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997; 89:2067–2078.

2. Norton AJ, Matthews J, Pappa V, et al. Mantle cell lymphoma: natural history defined in a serially biopsied population over a 20-year period. Ann Oncol. 1995; 6:249–256.

3. Cornes JS. Multiple lymphomatous polyposis of the gastrointestinal tract. Cancer. 1961; 14:249–257.

4. Meral M, Demirpençe M, Gönen C, et al. Diffuse gastrointestinal involvement of mantle cell lymphoma. Turk J Gastroenterol. 2008; 19:117–120.
5. Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003; 97:586–591.

6. Chung HH, Kim YH, Kim JH, et al. Imaging findings of mantle cell lymphoma involving gastrointestinal tract. Yonsei Med J. 2003; 44:49–57.

7. Nodit L, Bahler DW, Jacobs SA, Locker J, Swerdlow SH. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003; 34:1030–1034.

8. Orchard J, Garand R, Davis Z, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003; 101:4975–4981.

9. Tamura S, Ohkawauchi K, Yokoyama Y, et al. Non-multiple lymphomatous polyposis form of mantle cell lymphoma in the gastrointestinal tract. J Gastroenterol. 2004; 39:995–1000.

10. Hotta K, Oyama T, Kitamura Y, Tomori A, Miyata Y, Mitsuishi T. Mantle cell lymphoma presenting as multiple lymphomatous polyposis spreading widely to the small intestine and diagnosed by double-balloon endoscopy. Endoscopy. 2007; 39:Suppl 1. E347–E348.

11. Kikuchi T, Asano N, Noguchi T, et al. A case of primary gastric mantle cell lymphoma. Nihon Shokakibyo Gakkai Zasshi. 2009; 106:1168–1176.
12. Pitigoi D, Stoica V, Stoia R, Dobrea C, Becheanu G, Diculescu M. Gastric and colonic mantle cell lymphoma - incidental discovery. J Gastrointestin Liver Dis. 2009; 18:85–88.
13. Kim CH, Chun HJ, Kim TH, et al. Solitary primary gastric mantle cell lymphoma. Gut Liver. 2011; 5:527–531.

14. Weisenburger DD, Armitage JO. Mantle cell lymphoma-- an entity comes of age. Blood. 1996; 87:4483–4494.

15. Garcia-Conde J, Cabanillas F. Mantle cell lymphoma: a lymphoproliferative disorder associated with aberrant function of the cell cycle. Leukemia. 1996; 10:Suppl 2. s78–s83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download