Abstract
Background/Aims
Hybrid therapy was successful in eradicating Helicobacter pylori (H. pylori) according to previous reports. However, to the best of our knowledge, there have only been a few studies evaluating the optimal choice after hybrid failure. Hence, we aimed to evaluate the efficacy of moxifloxacin-containing triple therapy after hybrid therapy failure in H. pylori eradication.
Methods
Between January 2013 and March 2016, we retrospectively reviewed patients who underwent failed hybrid therapy, as first line treatment, in eradicating H. pylori (rabeprazole and amoxicillin b.i.d for 14 days, in addition to clarithromycin and metronidazole b.i.d for final 7 days). Then, we investigated the eradication rates of moxifloxacin-containing triple therapy (rabeprazole, amoxicillin b.i.d and moxifloxacin qd) as the second line of treatment. Intention-to-treat (ITT) and per-protocol (PP) analyses were used to determine the eradication rate. We evaluated the status of H. pylori by using 13 C-urea breath test 6 weeks after the final treatment. Moreover, compliance and adverse effects of each patient were analyzed.
Results
Among those who failed the initial hybrid therapy, 11 patients received moxifloxacin-containing triple therapy. The overall eradication rates, as determined by ITT and PP, were 72.7% (n=8/11) and 80% (n=8/10), respectively. The compliance rate was 100%, and there were no serious adverse effects.
Go to : 
References
1. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.
2. Kim JH, Kim HY, Kim NY, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001; 16:969–975.

3. Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007; 12:333–340.

4. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013; 13:104.

5. Lee JY, Kim N. Future trends of Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2014; 63:158–170.
6. Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: abdominal, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.
7. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.

8. Graham DY, Shiotani A. New concepts of resistance in the abdominal of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:321–331.
9. Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004; 10:668–671.
10. De Francesco V, Margiotta M, Zullo A, et al. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. J Antimicrob Chemother. 2007; 59:783–785.

11. Kim SE, Park MI, Park SJ, et al. Trends in Helicobacter pylori abdominal rates by first-line triple therapy and related factors in abdominal therapy. Korean J Intern Med. 2015; 30:801–807.
12. Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011; 16:139–145.
13. Hsu PI, Lin PC, Graham DY. Hybrid therapy for Helicobacter pylori infection: a systemic review and metaanalysis. World J Gastroenterol. 2015; 21:12954–12962.
14. Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J. High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr. 2007; 119:372–378.

15. Cheon JH, Kim N, Lee DH, et al. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006; 11:46–51.

16. Bago J, Pevec B, Tomić M, Marusić M, Bakula V, Bago P. Second-line treatment for Helicobacter pylori infection based on moxifloxacin triple therapy: a randomized controlled trial. Wien Klin Wochenschr. 2009; 121:47–52.

17. Hwang JJ, Lee DH, Lee AR, et al. Efficacy of 14-d vs 7-d moxi-floxacin-based triple regimens for second-line Helicobacter abdominal eradication. World J Gastroenterol. 2015; 21:5568–5574.
18. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple abdominal of Helicobacter pylori including CYP2C19 genotype and abdominal resistance. Dig Dis Sci. 2014; 59:1235–1243.
19. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011; 17:3971–3975.

20. Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2001; 15:1023–1029.

21. Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, ni-tazoxanide, and doxycycline versus triple therapy for the abdominal of Helicobacter pylori. Am J Gastroenterol. 2011; 106:1970–1975.
22. Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012; 27:504–509.

23. Nishizawa T, Suzuki H, Suzuki M, Takahashi M, Hibi T. Proton pump inhibitor-amoxicillin-clarithromycin versus proton pump inhibitor-amoxicillin-metronidazole as first-line Helicobacter abdominal eradication therapy. J Clin Biochem Nutr. 2012; 51:114–116.
24. Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010; 8:59–70.
25. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and abdominal antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
26. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy–the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999; 13:1047–1055.

27. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point abdominal of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.
28. Gong EJ, Yun SC, Jung HY, et al. Meta-analysis of first-line triple therapy for helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 2014; 29:704–713.

29. Current European concepts in the management of Helicobacter pylori infection. The Maastricht consensus report. European Helicobacter Pylori Study Group. Gut. 1997; 41:8–13.
30. Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008; 148:923–931.

31. Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The abdominal therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007; 56:1353–1357.
32. Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013; 47:383–388.
33. Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006; 119:217–224.

34. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009; 14:109–118.
35. McNicholl AG, Marin AC, Molina-Infante J, et al. Randomised abdominal trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut. 2014; 63:244–249.
36. Huang YK, Wu MC, Wang SS, et al. Lansoprazole-based abdominal and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012; 13:232–238.
37. Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010; 8:36–41.e1.

38. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidencebased medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014; 12:177–186.e3; Discussion e12-e13.

39. Hsu PI, Wu DC, Chen WC, et al. Randomized controlled trial abdominal 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014; 58:5936–5942.
40. Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013; 381:205–213.
41. Wu JY, Hsu PI, Wu DC, Graham DY, Wang WM. Feasibility of abdominal 14-day hybrid therapy while maintaining an excellent Helicobacter pylori eradication rate. Helicobacter. 2014; 19:207–213.
42. Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013; 18:129–134.
43. Zullo A, Scaccianoce G, De Francesco V, et al. Concomitant, abdominal, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013; 37:647–650.
44. De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 2014; 63(Pt 5):748–752.

45. Oh DH, Lee DH, Kang KK, et al. Efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol. 2014; 29:1171–1176.
46. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of abdominal resistance. Gastroenterology. 2013; 145:121–128.e1.
47. Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the abdominal of the second-line treatment regimens for Helicobacter abdominal infection. Scand J Gastroenterol. 2001; 36:690–700.
48. Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002; 16:1047–1057.
49. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory abdominal for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006; 28:6–13.
50. Keating GM, Scott LJ. Moxifloxacin: a review of its use in the abdominal of bacterial infections. Drugs. 2004; 64:2347–2377.
51. Edlund C, Beyer G, Hiemer-Bau M, Ziege S, Lode H, Nord CE. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand J Infect Dis. 2000; 32:81–85.
52. Kang JM, Kim N, Lee DH, et al. Second-line treatment for Helicobacter pylori infection: 10-day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007; 12:623–628.

53. Wu C, Chen X, Liu J, Li MY, Zhang ZQ, Wang ZQ. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple abdominal for second-line treatment of Helicobacter pylori infection: a metaanalysis. Helicobacter. 2011; 16:131–138.
54. Kang KK, Lee DH, Oh DH, et al. Helicobacter pylori eradication with moxifloxacin-containing therapy following failed first-line therapies in South Korea. World J Gastroenterol. 2014; 20:6932–6938.

55. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence abdominal report. Gut. 2012; 61:646–664.
Go to : 
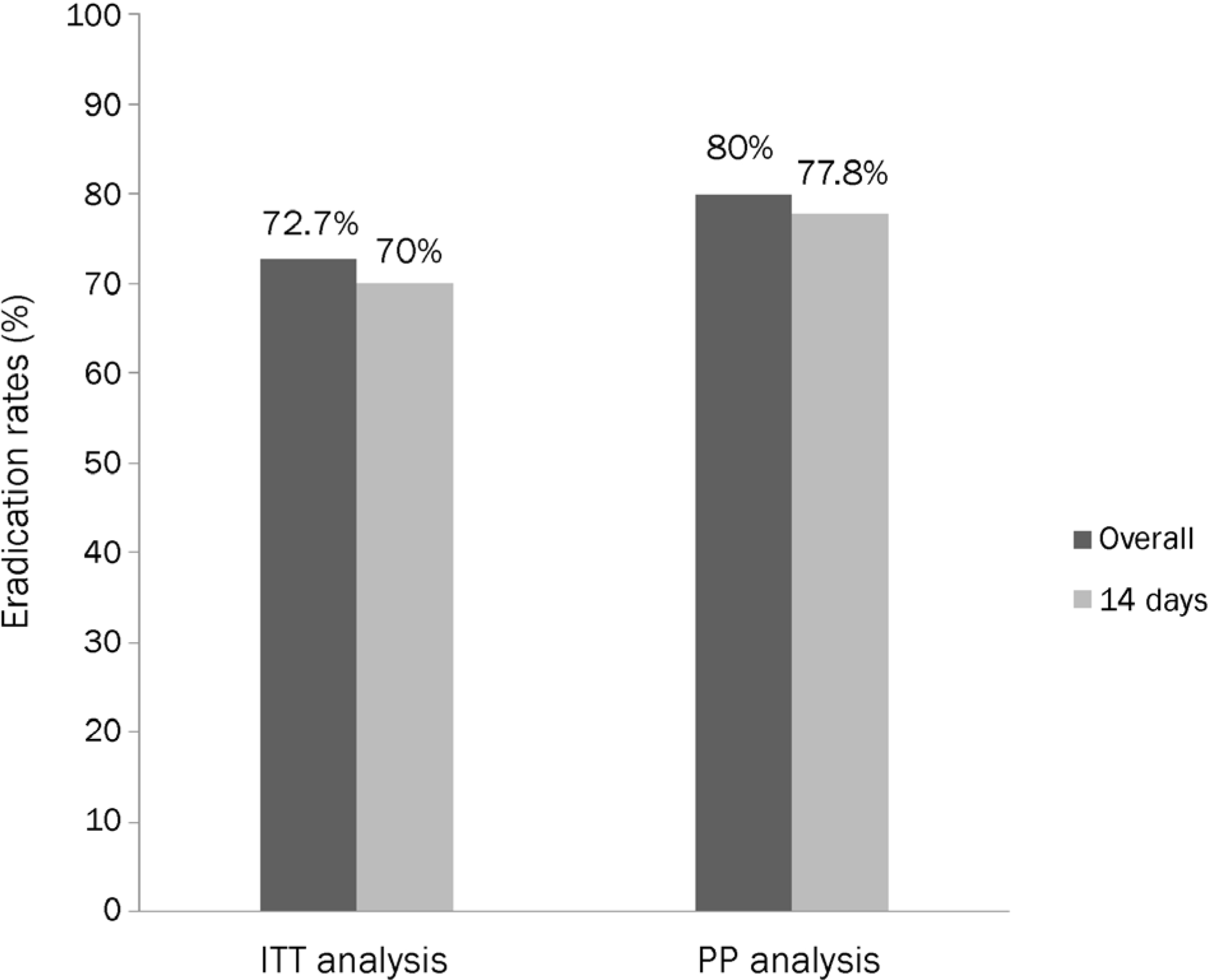 | Fig. 1.Eradication rates of the overall and 14-days moxifloxacin-containing triple therapy as the second line therapy after hybrid therapy failure. It is presented by intention-to-treat (ITT) and per-protocol (PP) analyses. |
Table 1.
Baseline Characteristics of Enrolled Patients
| Patient number | Sex | Age | Alcohol | Current smoker | Underlying diseases | EGD findings | Time intervals (days)a | Treatment duration (days)b | Treatment results b | Compliance b | Adverse eventsb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 66 | No | No | Osteoporosis | Gastritis | 47 | 14 | Eradicated | 100% | None |
| 2 | F | 71 | No | No | HTN | Gastritis | 84 | 7 | Eradicated | 100% | None |
| 3 | M | 63 | Yes | No | DM/HTN | Gastritis | 129 | 14 | Eradicated | 100% | None |
| 4 | M | 46 | Yes | Yes | None | Gastric ulcer | 97 | 14 | Eradicated | 100% | None |
| 5 | M | 62 | Yes | No | DM/HTN | Gastritis | 46 | 14 | Eradicated | 100% | None |
| 6 | F | 75 | No | No | None | Gastritis | 53 | 14 | Eradicated | 100% | None |
| 7 | M | 64 | Yes | No | DM/ALD | Gastritis | 102 | 14 | Follow up loss | ||
| 8 | F | 60 | No | No | None | Gastritis | 61 | 14 | Not eradicated | 100% | None |
| 9 | M | 67 | No | No | HTN/BPH | Not done | 45 | 14 | Not eradicated | 100% | None |
| 10 | F | 66 | No | No | None | Duodenal ulcer | 41 | 14 | Eradicated | 100% | Loose stool |
| 11 | F | 57 | No | No | None | Gastritis | 399 | 14 | Eradicated | 100% | None |
Table 2.
Comparison of Eradicated and Not Eradicated Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download