Abstract
Background/Aims
Helicobacter pylori (Hp) infection is an important risk factor for gastric carcinogenesis. Although several studies have investigated the effect of Hp eradication on the development of metachronous neoplasm after endoscopic resection of the gastric dysplasia, the evidence is still insufficient to make a clear conclusion. The aims of this study was to evaluate the risk factors for the development of metachronous neoplasm after endoscopic resection of gastric dysplasia and to investigate the effect of Hp eradication.
Methods
Between 2005 and 2011, a total of 887 patients underwent endoscopic resection for gastric dysplasia. Among them, 521 patients who had undergone tests for Hp infection and been followed-up for at least one year were included in the final analyses. Of the 292 Hp-positive patients, 116 patients were successfully eradicated, while 176 failed or did not undergo eradication.
Results
During a mean follow-up of 59.1 months (range 12–125 months), metachronous neoplasm had developed in 63 patients (12.1%, dysplasia in 38, carcinoma in 25). In multivariate analyses, age ≥65 (hazard ratio [HR]=2.247, 95% confidence interval [CI] 1.297–3.895), tumor size (HR=1.283, 95% CI 1.038–1.585), synchronous lesion (HR=2.341, 95% CI 1.244–4.405), family history of gastric cancer (HR=3.240, 95% CI 1.776–5.912), and smoking (HR=1.016, 95% CI 1.003–1.029) were risk factors for metachronous neoplasm after endoscopic resection of gastric dysplasia. However, Hp eradication was not associated with metachronous neoplasm (HR=0.641, 95% CI 0.297–1.384).
References
1. Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988; 48:3554–3560.
2. Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004; 291:187–194.
3. Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009; 137:1641–1648. e1-e2.

4. Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a metaanalysis. Gastric Cancer. 2016; 19:166–175.

5. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.

6. Choi J, Kim SG, Yoon H, et al. Eradication of helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014; 12:793–800.e1.
7. Maehata Y, Nakamura S, Fujisawa K, et al. Longterm effect of helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012; 75:39–46.

8. Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013; 62:1425–1432.

9. Chon I, Choi C, Shin CM, Park YS, Kim N, Lee DH. Effect of helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol. 2013; 61:307–312.
10. Shin SH, Jung DH, Kim JH, et al. Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS One. 2015; 10:e0143257. eCollection 2015.

11. Rugge M, Di Mario F, Cassaro M, et al. Pathology of the gastric antrum and body associated with helicobacter pylori infection in non-ulcerous patients: is the bacterium a promoter of intestinal metaplasia? Histopathology. 1993; 22:9–15.

12. Kobayashi M, Hashimoto S, Mizuno K, et al. Therapeutic or spontaneous helicobacter pylori eradication can obscure magnifying narrow-band imaging of gastric tumors. Endosc Int Open. 2016; 4:E665–E672.

13. Kawanaka M, Watari J, Kamiya N, et al. Effects of helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic treatment: analysis of molecular alterations by a randomised controlled trial. Br J Cancer. 2016; 114:21–29.

14. Niwa T, Tsukamoto T, Toyoda T, et al. Inflammatory processes triggered by helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010; 70:1430–1440.

16. Mori G, Nakajima T, Asada K, et al. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful helicobacter pylori eradication: results of a largescale, multicenter cohort study in Japan. Gastric Cancer. 2016; 19:911–918.

17. Nakajima T, Maekita T, Oda I, et al. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev. 2006; 15:2317–2321.

18. Bae SE, Jung HY, Kang J, et al. Effect of helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. 2014; 109:60–67.

Fig. 1.
Cumulative incidence rate of metachronous tumor (Kaplan-Meier survival curve). There was no significant difference of cumulative incidence rate of metachronous tumor in accordance with the status of Helicobacter pylori (Hp) infection.
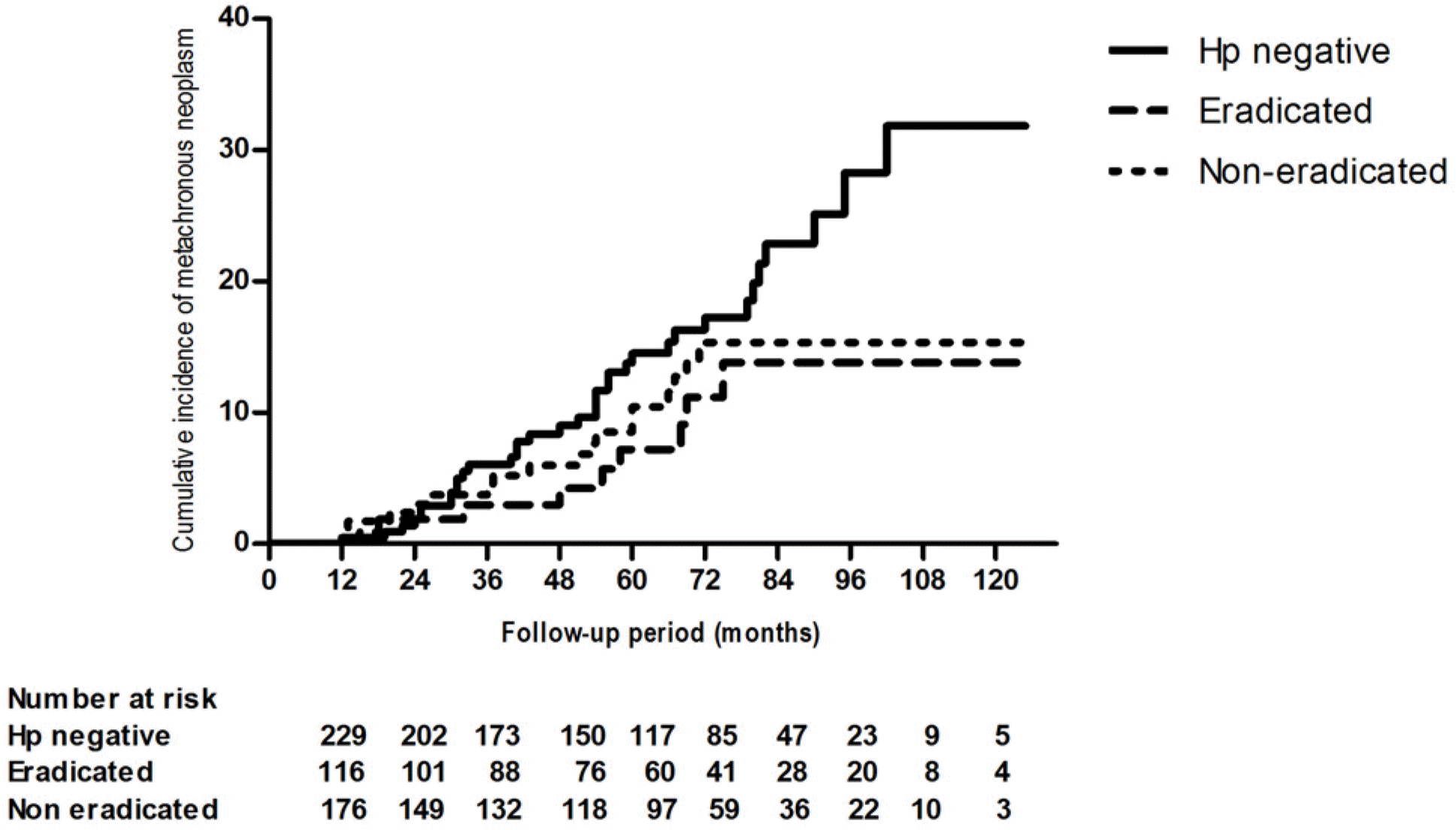
Table 1.
Baseline Characteristics of the Study Population
Table 2.
Associated Factors for Metachronous Neoplasm of Gastric Dysplasia
| Metachronous neoplasm (−)(n=458) | Metachronous neoplasm (+)(n=63) | p-value | HR a | 95% CI a | |
|---|---|---|---|---|---|
| Age | 0.004 b | ||||
| <65 | 297 (64.8) | 29 (46.0) | Reference | ||
| ≥65 | 161 (35.2) | 34 (54.0) | 2.425 | 1.474–3.987 | |
| Sex | 0.059 b | ||||
| Female | 180 (39.3) | 17 (27.0) | Reference | ||
| Male | 278 (60.7) | 46 (73.0) | 1.583 | 0.907–2.762 | |
| Hp status | 0.104 b | ||||
| No infection | 194 (42.4) | 35 (55.6) | Reference | ||
| Eradicated | 107 (23.4) | 9 (14.3) | 0.498 | 0.239–1.036 | |
| Not eradicated | 157 (34.3) | 19 (30.2) | 0.709 | 0.406–1.240 | |
| Location | 0.230 b | ||||
| Upper third | 9 (2.0) | 3 (4.8) | Reference | ||
| Middle third | 186 (40.6) | 29 (46.0) | 0.527 | 0.160–1.734 | |
| Lower third | 263 (57.4) | 31 (49.2) | 0.388 | 0.118–1.270 | |
| Size (cm) | 1.37±0.93 | 1.88±1.32 | <0.001 c | 1.430 | 1.192–1.714 |
| Synchronous lesion(s) | <0.001 b | ||||
| No | 416 (90.8) | 48 (76.2) | Reference | ||
| Yes | 42 (9.2) | 15 (23.8) | 2.791 | 1.562–4.988 | |
| Histological grade | 0.102 b | ||||
| Low grade dysplasia | 334 (72.9) | 52 (82.5) | Reference | ||
| High grade dysplasia | 124 (27.1) | 11 (17.5) | 0.539 | 0.160–1.734 | |
| Intestinal metaplasia | 0.017 b | ||||
| No | 52 (11.5) | 1 (1.6) | Reference | ||
| Yes | 402 (88.5) | 61 (98.4) | 7.956 | 1.103–57.401 | |
| Family history of GC | 0.001 b | ||||
| No | 393 (87.3) | 45 (71.4) | Reference | ||
| Yes | 57 (12.7) | 18 (28.6) | 2.755 | 1.594–4.761 | |
| Smoking (pack-year) | 8.42±15.69 | 15.89±22.25 | 0.001 c | 1.017 | 1.006–1.029 |
| Alcohol (g/d) | 9.35±21.38 | 16.08±30.11 | 0.031 c | 1.006 | 0.998–1.014 |
| Follow up period (months) | 60.66±29.41 | 47.84±22.80 | 0.001 c | − | − |
Table 3.
Risk Factors for Metachronous Cancer after Endoscopic Resection of Gastric Dysplasia
| HR a | 95% CI a | |
|---|---|---|
| Age ≥65 | 2.247 | 1.297–3.895 |
| Male sex | 1.047 | 0.557–1.970 |
| Hp status | ||
| No infection | Reference | |
| Eradicated | 0.641 | 0.297–1.384 |
| Not eradicated | 1.103 | 0.601–2.026 |
| Size (cm) | 1.283 | 1.038–1.585 |
| Synchronous lesion(s) | 2.341 | 1.244–4.405 |
| Intestinal metaplasia | 4.884 | 0.669–35.663 |
| Family history of GC | 3.240 | 1.776–5.912 |
| Smoking (pack-year) | 1.016 | 1.003–1.029 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download