This article has been corrected. See "Correction: The Value of Computed Tomography in Preoperative N Staging of Early Gastric Cancer Meeting the Endoscopic Resection Criteria" in Volume 70 on page 157.
Abstract
Background/Aims
This study evaluated the value of abdominal computed tomography (CT) in preoperative N staging of early gastric cancers (EGCs) within standard and expanded indications of endoscopic resection (ER) and investigated the factors affecting accuracy.
Methods
Between March 2009 and March 2016, a total of 268 patients with EGC within the standard and expanded indications of ER underwent preoperative abdominal CT and surgical gastrectomy with lymph node (LN) dissection. Preoperative N staging of CT was compared with the pathologic result.
Results
The accuracy of N staging for EGCs within the standard and expanded indications was 86.1% (235/268). There was no LN metastasis in patients with cN1 in CT staging. LN metastasis was found in 7 patients with EGCs that met the expanded ER indication and cN0 in CT staging. According to the univariate analysis, ulcers, including scars, were associated with the false positive of lymph node metastasis in abdominal CT (odds ratio 3.56; 95% confidence interval 1.56–8.15).
Go to : 
References
1. Park JI, Jin SH, Bang HY, Paik NS, Moon NM, Lee JI. Survival rates after operation for gastric cancer: fifteen-year experience at a Korea Cancer Center Hospital. J Korean Gastric Cancer Assoc. 2008; 8:9–19.

2. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009; 69:1228–1235.

3. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009; 58:331–336.

4. Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007; 66:693–700.
5. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225.

6. Lee HH, Yoo HM, Song KY, Jeon HM, Park CH. Risk of limited lymph node dissection in patients with clinically early gastric cancer: indications of extended lymph node dissection for early gastric cancer. Ann Surg Oncol. 2013; 20:3534–3540.

7. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
8. Lee DH, Ko YT, Park SJ, Lim JW. Comparison of hydro-US and spiral CT in the staging of gastric cancer. Clin Imaging. 2001; 25:181–186.

9. Fukuya T, Honda H, Kaneko K, et al. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997; 21:73–81.

10. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–S43.
11. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma:3rd English edition. Gastric Cancer. 2011; 14:101–112.
12. Mönig SP, Zirbes TK, Schröder W, et al. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol. 1999; 173:365–367.

13. Lee JH, Kim JG, Jung HK, et al. Synopsis on clinical practice guideline of gastric cancer in Korea: an evidence-based approach. Korean J Gastroenterol. 2014; 63:66–81.

14. Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011; 73:942–948.

15. Jang JS, Choi SR, Qureshi W, et al. Longterm outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009; 44:1315–1322.

16. Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver. 2015; 9:174–180.

17. Aoyagi K, Koufuji K, Yano S, et al. Two cases of early gastric carcinoma with synchronous liver metastasis. Kurume Med J. 2003; 50:53–56.

18. Choi JY, Kim JI, Choi YC, Jun SY. Two cases of histopathologically advanced (stage IV) early gastric cancer. Korean J Gastroenterol. 2005; 45:64–67.
19. Hwang EJ, Jang JY, Kim YW, et al. A case of early gastric cancer with solitary metastasis to the pleura. Clin Endosc. 2013; 46:666–670.

20. Yamada T, Sugiyama H, Ochi D, et al. Risk factors for submucosal and lymphovascular invasion in gastric cancer looking indicative for endoscopic submucosal dissection. Gastric Cancer. 2014; 17:692–696.

21. Feng H, Wang Y, Cao L, et al. Lymph node metastasis in differentiated-type early gastric cancer: a single-center retrospective analysis of surgically resected cases. Scand J Gastroenterol. 2016; 51:48–54.

22. Kwak CS, Lee HK, Cho SJ, et al. Analysis of clinicopathological factors associated with lymph node metastasis in early gastric cancer review of 2,137 cases. J Korean Cancer Assoc. 2000; 32:674–681.
23. Xue N, Huang P, Aronow WS, et al. Predicting lymph node status in patients with early gastric carcinoma using double contrastenhanced ultrasonography. Arch Med Sci. 2011; 7:457–464.

24. Yang F, Zhang S, Xin XJ, Wei X, Xu Y. Application of three-dimensional ultrasonography to assess the abdominal lymph node metastasis of gastric carcinoma. Zhonghua Zhong Liu Za Zhi. 2016; 38:385–388.
25. Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005; 46:1582–1588.
Go to : 
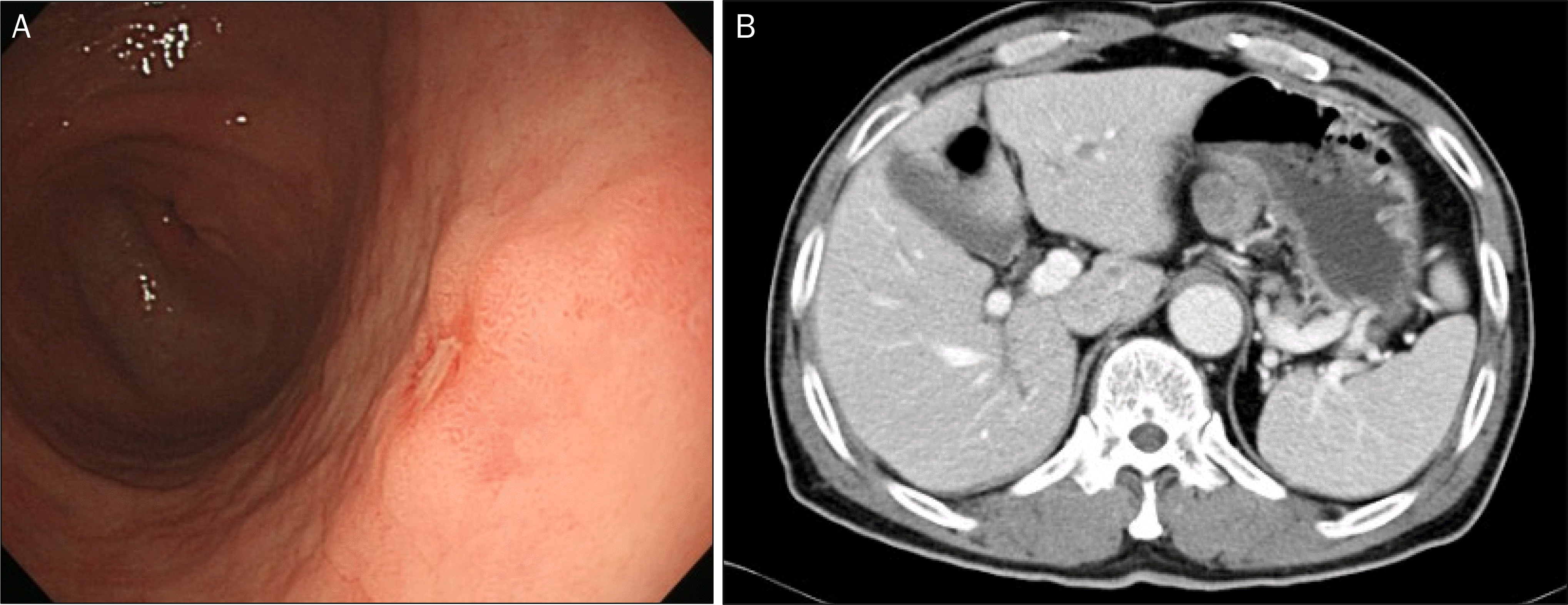 | Fig. 1.Early gastric cancer (well differentiated adenocarcinoma, depth of invasion limited to the lamina propria, size 0.4×0.2 cm) with no lymph node involvement (32 regional lymph nodes). (A) Conventional endoscopy shows a flat and elevated lesion with erosion on the surface. (B) Computed tomography shows a lymph node enlargement (4.0×2.3 cm) on the left gastric area. |
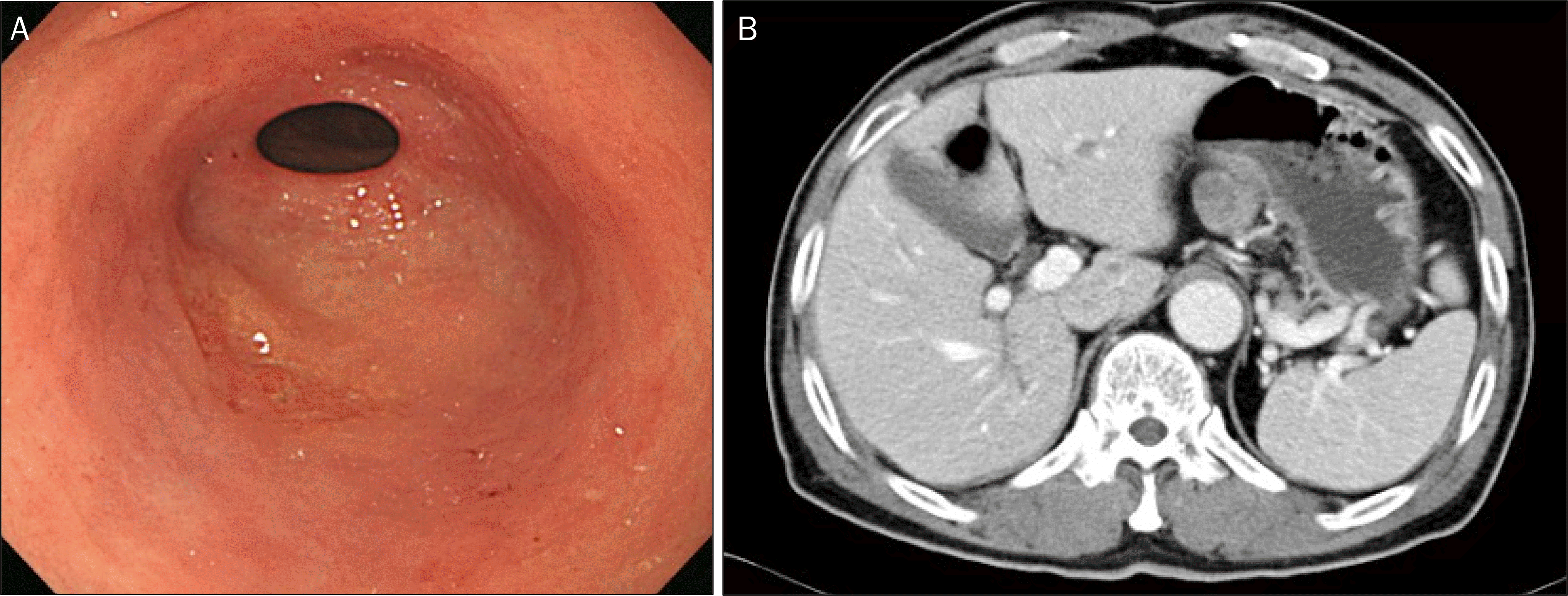 | Fig. 2.Early gastric cancer (mixed histology consisted of 70% moderately differentiated and 30% poorly differentiated adenocarcinoma, depth of invasion limited to the mucosa, size 2.5×1.7 cm) with lymph node involvement (metastasis to 1 out of 22 regional lymph nodes). (A) Conventional endoscopy shows a depressed scar lesion with irregular surface. (B) Computed tomography shows no perigastric lymph node enlargement. |
Table 1.
Baseline Characteristics of Patients with Early Gastric Cancers
Table 2.
LNM according to the N Staging of CT and Criteria of Categories
Table 3.
Univariate Analysis of the Endoscopic Features Associated with Lymph Node Metastasis
Table 4.
Univariate Analysis of the Endoscopic Features Associated with Lymph Node Enlargement on an Image of Computed Tomography
Table 5.
Cases of Early Gastric Cancer with Lymph Node Metastasis that Met the Endoscopic Resection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download