Abstract
Pancreatic pseudocyst is a common complication of acute pancreatitis. Pseudocysts are commonly observed in the lesser sac and retroperitoneum; they are rarely seen in the liver. Herein, we report a case of intrahepatic pseudocyst, complicated by asymptomatic groove pancreatitis, that has successfully been treated with hepatic resection. A 70-year-old woman was referred to our hospital with severe upper abdominal pain. Abdominal computed tomography scan showed 11×10 cm sized cystic lesion in the left lateral section of the liver. Appearance of the pancreas was relatively normal. Endoscopic aspiration revealed a high level of amylase in the cystic fluid. After endoscopy, signs of peritonitis were observed; then, a left hemihepatectomy was performed. Pathologic examination revealed an intrahepatic pancreatic pseudocyst. The presence of intrahepatic cystic lesion in patients with suspected pancreatitis should raise the suspicion of intrahepatic pseudocyst. Intrahepatic pancreatic pseudocysts may be the only clinical manifestation even without an episode of acute pancreatitis.
Pancreatic pseudocyst is defined as a collection of pancreatic juice enclosed by a wall of non-epithelialized granulation tissue or fibrotic capsule. Pancreatic pseudocyst is a common complication of acute pancreatitis that can occur anywhere in the abdomen. Most commonly, they occur around the pancreas, lesser sac, and retroperitoneum. However, they have been reported to be found distant from the pancreas, such as in the mediastinum or scrotum.12 Intrahepatic location of the pancreatic pseudocyst is a very rare event. To date, only about 50 cases of intrahepatic pancreatic pseudocysts (IHPPs) have been reported.3 Herein, we report a case of IHPP in the left hepatic lobe that has successfully been treated with left hemihepatectomy.
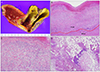
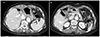
A 70-year-old woman had been hospitalized in another hospital for suspected acute pancreatitis. She visited the hospital with upper abdominal pain that last for 2 days. The initial levels of serum amylase and lipase were 669 U/L and 840 U/L, respectively. Abdominal computed tomography (CT) at the other hospital revealed a 12×10×10 cm sized cystic tumor between the liver and pancreatic body; however, the appearance of the pancreas was normal (Fig. 1). On the following day, she was referred to our hospital due to severely aggravated upper abdominal pain, despite medical treatment. She had no past history of pancreatitis or alcohol consumption. A physical examination showed mild epigastric tenderness without signs of peritoneal irritation. Laboratory studies revealed white blood cell count of 12,000/mm3, hemoglobin level of 13.3 g/dL, and platelet count of 250,000/mm3. The level of serum amylase and lipase were elevated to 484 U/L and 215 U/L, respectively. The serum levels of aspartate aminotransferase (31 U/L), alanine aminotransferase (26 U/L), alkaline phosphatase (48 U/L), and total bilirubin (0.92 mg/dL) were within normal limits. Abdominal CT scan showed a 10.3×6.7×11.7 cm sized cystic mass in the left lateral section of the liver (Fig. 2A). Internal sepation or irregular papillary growth was not observed within the cystic lesion. The porto- mesenteric junction was collapsed by mass effect of the cystic lesion (Fig 2B). The cystic lesion was not communicated with the pancreas. The boundary between the pancreatic body and cystic mass was clearly delineated (Fig. 2B). Appearance of the pancreas on the CT scan was relatively normal; however, soft tissue straining was observed around the duodenum, which resulted in the suspicion of groove pancreatitis (Fig 2B). The Glisson pedicle in the umbilical fissure was swollen remarkably (Fig. 2C). Endoscopic ultrasonography (EUS)-guided transgatric internal drainage was carried out due to the suspicion of infected hepatic cyst or a pancreatic pseudocyst. An EUS demonstrated a huge hypoechoic lesion with internal echogenicity (Fig. 3A); however, no solid component or mural nodule was observed. The cystic lesion was punctured with a 19-gauge needle; then, dark green colored turbid fluid was aspirated. The tract was dilated with 6-8 mm sized CRE™ balloon dilatation catheters (Boston scientific corporation, Boston, MA, USA) and a 7-Fr bipigtailed plastic stent was deployed (Fig. 3B). Cystic fluid analysis revealed high levels of amylase (21,200 U/L), lipase (1,392 U/L), and total bilirubin (5.18 mg/dL). The carcinoembryonic antigen level of the cystic fluid was 70.68 ng/mL. Unfortunately, after the endoscopic procedure, abdominal pain was aggravated. On physical examination, severe epigastric tenderness, rebound tenderness, and rigidity were observed. The rupture of the cystic lesion was suspected; therefore, an emergency operation was performed. According to operative findings, a huge cystic mass was located in the left lateral section of the liver. Cystic mass contained brown turbid fluid and necrotic tissues, and cystic fluid spilled into the lesser sac. There was no communication between the cystic tumor and pancreas. Porta hepatis, Glisson pedicle in the umbilical portion, and falciform ligament were swollen remarkably and filled with necrotic fatty tissues (Fig. 4). A left hemihepatectomy was performed. Grossly, an intrahepatic unilocular cyst containing a ragged inner surface and thick fibrous wall was observed (Fig. 5A). Pathological examination revealed a pseudocystic nature. The cystic lesion was devoid of an epithelial lining (Fig. 5B). The lining of the pseudocyst was composed of granulation tissue, inflammatory cells, and fibrotic tissue (Fig. 5C). The falciform ligament was composed of marked necrosis with fat necrosis and acute inflammatory cells (Fig. 5D). They seem to be common findings in acute pancreatitis. Inclusively, the final diagnosis confirmed intrahepatic pancreatic pseudocyst. The patient was discharged without any complication on postoperative days 11. No complication was observed during the follow-up period. Twenty-eight months after the operation, the patient was disease-free (Fig. 6).
Only about 50 cases of IHPP following pancreatitis have been described in the literature.3 In our case, the patient had no history or symptom of acute pancreatitis, and there was no obvious evidence of acute pancreatitis on an image study, except slightly elevated serum amylase and lipase levels. Despite normal appearance of the pancreas, soft tissue of the porta hepatis and faliciform ligament was markedly necrotized; and there was an occurrence of huge IHPP. IHPP was the only clinical manifestation without any obvious symptom of preceding acute pancreatitis. Patient's symptom ―upper abdominal pain― was related to IHPP. It is presumed that subclinical groove pancreatitis have already existed. Demeusy et al. have reported that of the 54 cases of IHPP since the 1970s, the most common location of IHPP was the left hepatic lobe (48%).3 CT is generally considered as a modality of choice for the diagnosis of the IHPP. However, diagnosis of IHPP can be difficult sometimes due to its rarity, especially in cases without any history of pancreatitis or obvious findings of pancreatitis. IHPPs should be differentiated from other conditions, such as intrahepatic biliary dilatation, peribiliary cyst, complicated hepatic cyst, liver abscess, biloma, echinococcal cyst, mucinous cystic neoplasm, and intraductal mucinous neoplasm of the bile duct. Cystic fluid analysis, which demonstrated a high amylase content, can be a definitive diagnosis of IHPPs.3 In previous reports, almost all cases were treated with percutaneous drainage or endoscopic procedure.45678 In our case, there was no obvious evidence of acute pancreatitis on abdominal CT; instead the scan showed normal appearance of the pancreas, huge intrahepatic cystic tumor, and enlarged lymph nodes of porta hepatis. Hence, differential diagnosis was required, including complicated heaptic cystic tumors, such as infected hepatic cyst, mucinous cystic neoplasm, or intraductal papillary neoplasm of the bile duct. Cystic fluid analysis has limited sensitivity in differentiating the hepatic cystic lesion. Surgical resection is ultimately necessary to confirm the diagnosis.9 Cystic fluid analysis revealed a high level of amylase content, suggesting IHPP. EUS-guided transgastric internal drainage could be a definitive treatment modality. However, after endoscopic procedure, signs of peritonitis and clinical deterioration were observed. Therefore, we decided to perform the operation.
There are two mechanisms proposed to explain the pathophysiology of IHPP.410111213 The first mechanism is consisted of pancreatic enzyme released following acute pancreatitis into the lesser sac spreading along the lesser omentum and gastrohepatic ligaments. This mechanism explains why IHPPs are frequently observed in the left lobe. The second mechanism is the spreading of pancreatic enzyme from the pancreatic head into the hepatoduodenal ligament and porta hepatis, along the portal vein and its branches. In our case, marked swelling and necrosis were observed in hepatoduodenal ligament, umbilical portion, and falciform ligament. However, there was no obvious finding of acute pancreatitis at the pancreatic body and tail or communication between the cystic mass and the pancreas. Therefore, the second mechanism is more suitable for explaining the pathophysiology in this case.
In conclusion, IHPPs should be considered when a huge intrahepatic cystic lesion is found in patients with recent episodes of pancreatitis, even if the appearance of pancreas is normal on an imaging study. The high level of amylase on cystic fluid analysis plays a key role in the diagnosis of IHPPs. For IHPPs, drainage procedure or surgical resection can be applied as a definitive treatment modality.
Figures and Tables
Fig. 1
(A, B) Abdominal computed tomography scan revealed a huge cystic lesion in the lesser sac (white arrow). The appearance of the pancreas was normal (yellow arrow).

Fig. 2
(A) Follow-up abdominal computed tomography scan demonstrated a huge cystic mass arising from left lateral section of the liver. (B) Porto-mesenteric junction collapsed by the mass effect of cystic tumor (yellow arrows). The border between the pancreas and cystic mass was clearly demarcated (yellow arrow heads). Soft tissue stranded around the duodenum was seen, suggesting groove pancreatitis (white arrows). (C) Prominent periportal edema in the umbilical fissure was observed (white arrows).
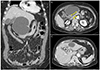
Fig. 3
(A) Endoscopic ultrasonography revealed a huge hypoechoic lesion with internal echogenicity without any solid components or mural nodes. (B) A 7-Fr bipigtailed plastic stent was deployed.

Fig. 4
(A) A huge cystic mass arising from the left lateral section of the liver was seen. (B) The falciform ligament and Glisson pedicle were swollen remarkably and filled with necrotic fatty tissues (arrows).
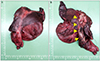
Fig. 5
(A) Gross features: an unilocular cyst containing a ragged inner surface and thick fibrous wall. (B) The cyst is devoid of an epithelial lining (H&E stain, ×40). (C) The lining of the cyst is composed of granulation tissue, inflammatory cells, and fibrous tissue (H&E stain, ×100). (D) Marked necrosis, fat necrosis, and acute inflammatory cells are observed in the falciform ligament (H&E stain, ×100).
References
1. Bardia A, Stoikes N, Wilkinson NW. Mediastinal pancreatic pseudocyst with acute airway obstruction. J Gastrointest Surg. 2006; 10:146–150.
2. Skouras C, Skouras T, Pai M, Zacharakis E, Spalding D. Inguinoscrotal extension of a pancreatic collection: a rare complication of pancreatitis-case report and review of the literature. Updates Surg. 2013; 65:153–159.
3. Demeusy A, Hosseini M, Sill AM, Cunningham SC. Intrahepatic pancreatic pseudocyst: a review of the world literature. World J Hepatol. 2016; 8:1576–1583.
4. Bhasin DK, Rana SS, Chandail VS, et al. An intra-hepatic pancreatic pseudocyst successfully treated endoscopic transpapillary drainage alone. JOP. 2005; 6:593–597.
5. Bhasin DK, Rana SS, Nanda M, et al. Endoscopic management of pancreatic pseudocysts at atypical locations. Surg Endosc. 2010; 24:1085–1091.
6. Baydar B, Cantürk F, Alper E, et al. Intrahepatic localization of pancreatic pseudocyst: a case report. Turk J Gastroenterol. 2013; 24:447–449.
7. Martínez-Sanz N, González-Valverde FM, Vicente-Ruiz M, Pastor-Pérez P, Ruiz-Marín M, Albarracín-Marín-Blázquez A. Intrahepatic pancreatic pseudocyst: a case report. Rev Esp Enferm Dig. 2015; 107:249–250.
8. Yi CY, Na GJ, Baek HC, et al. A case of intrahepatic pseudocyst complicating acute pancreatitis. Korean J Gastroenterol. 2008; 51:56–59.
9. Marrero JA, Ahn J, Rajender Reddy. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014; 109:1328–1347.
10. Casado D, Sabater L, Calvete J, et al. Multiple intrahepatic pseudocysts in acute pancreatitis. World J Gastroenterol. 2007; 13:4655–4657.
11. Okuda K, Sugita S, Tsukada E, Sakuma Y, Ohkubo K. Pancreatic pseudocyst in the left hepatic lobe: a report of two cases. Hepatology. 1991; 13:359–363.
12. Siegelman SS, Copeland BE, Saba GP, Cameron JL, Sanders RC, Zerhouni EA. CT of fluid collections associated with pancreatitis. AJR Am J Roentgenol. 1980; 134:1121–1132.
13. Ancel D, Lefebvre M, Peyrin-Biroulet L, et al. Pancreatic pseudocysts of the right hepatic lobe during acute biliary pancreatitis. Gastroenterol Clin Biol. 2005; 29:743–745.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download