Abstract
An isolated pyogenic pancreatic abscess (IPPA) without pancreatitis is extremely rare but can occur in patients with uncontrolled diabetes. This pathologic condition poses a clinical challenge in diagnosis and management because it can be confused easily with a malignancy. Endoscopic ultrasound (EUS) may be a useful diagnostic modality for indeterminate pancreatic lesions and IPPA. Here, we report two cases with elevated carbohydrate antigen 19-9 levels and pancreatic masses on cross sectional imaging. The patients were subsequently diagnosed with IPPA by EUS. EUS-guided drainage was performed successfully and the patients' clinical symptoms and radiologic findings improved. In our experience, EUS and EUS-guided drainage are crucial steps for the diagnosis and management of patients with an indeterminate pancreatic lesion. In addition, EUS-guided drainage has excellent technical and clinical outcomes for the treatment of IPPA.
An isolated pyogenic pancreatic abscess (IPPA) without pancreatitis is rare and usually caused by tuberculosis, salmonellosis, or underlying diabetes.123 Surgical debridement or computed tomography (CT)-guided percutaneous drainage remains the standard treatment for a pancreatic abscess. Recently, fine needle aspiration (FNA) or drainage catheter placement performed by endoscopic ultrasound (EUS) has become a treatment option because they are more effective and less invasive. Herein, we report two cases of IPPA in patients with underlying diabetes mellitus (DM) who were treated successfully by EUS-guided drainage.
Case 1: A 62-year-old man with DM presented with a history of fever, nausea, and pain in the epigastrium for 1 month. He denied any smoking or alcohol consumption. Laboratory studies revealed an elevation of C-reactive protein 43.52 mg/L, glucose 244 mg/dL, hemoglobin A1c 8.5%, and carbohydrate antigen 19-9 (CA 19-9) 93 U/mL, but all other findings were unremarkable. An abdominal CT and magnetic resonance cholangiopancreatography showed a 4.8 cm ill-defined low density mass in the pancreas head with regional lymph node enlargement (Fig. 1A, B). After obtaining informed consent, EUS via a longitudinal echoendoscope (Olympus GF-UCT 260, Tokyo, Japan) revealed a 4 cm anechoic lesion containing echogenic materials. Color Doppler was applied to identify any interposing vessels. EUS-FNA using a 20-G EUS needle (Echotip ProCore® HD Ultrasound biopsy needle; Wilson-Cook Medical Inc., Bloomington, IN, USA) was performed (Fig. 1C) and a thick, opaque, purulent fluid consistent with an abscess was aspirated (Fig. 1D). A naso-abscess drainage catheter (5 Fr) was also placed (Fig. 1E) and removed 5 days later when the amount of drainage had decreased significantly. The fluid aspiration tested positive for Klebsiella pneumonia and negative for acid-fast staining. The patient recovered uneventfully and was discharged after 10 days on antibiotics. A repeated CT scan revealed complete resolution of the abscess (Fig. 1F).
Case 2: A 51-year-old man was admitted to our department complaining of abdominal pain, 5 kg weight loss, fever up to 38℃, and chills. He had a history of DM, hypertension, chronic kidney disease, and complete atrioventricular block treated with a pacemaker. The physical examination revealed mild direct tenderness of the epigastrium. A blood test performed after admission revealed the following: white bleed cell count, 6,620/mm3 (69.3% neutrophil); hemoglobin, 11.3 g/dL; aspartate aminotransferase, 23 U/L; alanine aminotransferase, 22 U/L; alkaline phosphatase, 252 IU/L; total bilirubin, 0.86 mg/dL; amylase, 54 U/L; lipase, 20 U/L; blood urea nitrogen, 58.8 mg/dL; creatinine, 2.57 mg/dL; glucose, 312 mg/dL; hemoglobin A1c, 8.8%; C-reactive protein, 63.12 mg/L; and CA 19-9, 97 U/mL. Non-contrast enhanced abdominal CT showed an irregular exophytic mass in the pancreas uncinate process (Fig. 2A). EUS revealed an ill-defined, approximately 2.8×2.3 cm hypo-echoic mass-like lesion with floating echogenic materials in the pancreas uncinate process. EUS-FNA was performed using a 22-G EUS needle (Echotip® Ultra Endoscopic Ultrasound needle; Wilson-Cook Medical Inc., Bloomington, IN, USA) (Fig. 2B) and a pus-like discharge was removed. Klebsiella pneumonia was isolated from the pus, which was negative for tuberculosis or a malignancy upon the cytopathologic examinations. Seven days after the procedure, the patient developed a fever and leukocytosis. The follow-up EUS showed a remnant abscess in the uncinate process, which required further intervention. A 2nd EUS-guided drainage was performed successfully (Fig. 2C). Since then, the patient has been asymptomatic. After 3 months, abdominal CT demonstrated successful resolution of the pancreatic abscess (Fig. 2D).
In general, a pancreatic abscess is a complication of a pancreatic pseudocyst infection that is correlated with high mortality due to acute pancreatitis, primary and recurrent chronic pancreatitis, abdominal trauma, or surgery. Tuberculosis, scrub typhus, and salmonellosis are other rare causative factors of pancreatic abscesses.123 In the present two cases, there was no definite etiology, but underlying uncontrolled DM. DM is correlated with depressed cellmediated immunity and disorders of humoral immunity. Consequently, DM increases the frequency and severity of various types of bacterial infections, including abscesses.45 Several reports have demonstrated underlying diabetes as a predisposing factor for pancreatic abscesses secondary to Klebsiella pneumonia, similar to these two cases.6
On abdominal CT or magnetic resonance imaging, an isolated pancreatic solid or cystic lesion with marked elevated CA 19-9 is often confused with various pancreatic pathologic conditions, such as adenocarcinoma, cystadenocarcinoma, neuroendocrine tumor, or pancreatic pseudocysts. EUS can provide a detailed ultrasound image, and may help reduce the diagnostic errors and offer a precise diagnosis of an indeterminate pancreatic lesion on cross-sectional abdominal imaging. A pathologic evaluation of the lesion is also essential and EUS-guided FNA may be the preferred diagnostic modality because of its high sensitivity and specificity for identifying the etiology of pancreatic masses.7
In the second case, intravenous contrast for CT scans was unavailable due to renal function impairment; thus, the image quality was suboptimal. The exophytic solid mass of the pancreas uncinate process on non-contrast enhanced CT with a marked elevated CA 19-9 level mimicked a pancreatic malignancy. On the other hand, on EUS, a low echoic lesion with an irregular margin and floating material was more compatible with an abscess and the abscess fluid was subsequently aspirated via EUS-FNA. Klebsiella pneumonia was found with no evidence of malignancy.
The treatment of pancreatic abscesses is complicated.8 Surgical approaches are associated with significant morbidity and mortality.9 The lesion in the pancreas may be difficult to target with CT or ultrasound-guided percutaneous drainage. Since the advent of linear-array EUS scopes with the evolving EUS techniques and accessories, EUS-guided abscess drainage of the liver, pelvic cavity, or pancreas has been investigated with a high clinical success rate achieved and a low rate of adverse events.
This report describes the safe and successful EUS-guided drainage of IPPA in two patients. The lesion showed a remarkable resolution following the EUS-guided drainage with normalization of CA 19-9. EUS-guided drainage has several advantages: (1) excellent visualization of the lesion on the pancreas with the surrounding structures; (2) access to the nearest site of the abscess while avoiding puncturing large vessels under Doppler ultrasound guidance; (3) direct passage of the needle into the abscess pocket through the gastric or duodenal wall alone; and (4) avoiding transcutaneous infections. Although more well-designed studies will be required, EUS-guided drainage is a safer and more effective alternative to surgery or percutaneous drainage.10
In conclusion, EUS and EUS-guided drainage should be considered as a crucial step in the diagnosis and treatment of indeterminate pancreatic lesions. In addition, as the imaging findings may mimic neoplasms, clinicians should consider pancreatic abscesses in a differential diagnosis, particularly in patients with DM.
Figures and Tables
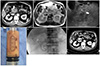 | Fig. 1(A) Transverse abdominal CT scans shows an ill-defined low attenuating mass (white arrow) along the pancreatic head. (B) T-2 weighted images demonstrate high signal intensity (black arrow) in the central portion of the mass. (C) A 20-G FNA needle (white arrow) entering the ill-defined low echoic lesion for aspiration is visible. (D) Yellowish pus discharge from the aspiration fluid collected by EUS-FNA. (E) Fluoroscopic view, demonstrating a naso-abscess catheter (5 Fr) placed into the abscess cavity through the duodenal wall. (F) Follow-up CT shows the resolution of an isolated pyogenic pancreatic abscess. CT, computed tomography; FNA, fine needle aspiration; EUS, endoscopic ultrasound. |
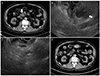 | Fig. 2(A) Non-contrast enhanced abdominal CT showing an exophytic mass in the uncinate process of the pancreas. (B) On EUS, a heterogeneous low echoic lesion with floating echogenic materials (white arrow) is visible. (C) EUS image of the 2nd abscess drainage using a 22 gauge needle in the remaining abscess cavity. (D) After 3 months, abdominal CT shows the resolved state of the exophytic pancreatic mass. CT, computed tomography; EUS, endoscopic ultrasound. |
References
1. Liu Q, He Z, Bie P. Solitary pancreatic tuberculous abscess mimicking pancreatic cystadenocarcinoma: a case report. BMC Gastroenterol. 2003; 3:1.
2. Arya M, Arya PK. Pancreatic abscess caused by s. typhi. Indian J Med Microbiol. 2001; 19:18–19.
3. Yi SY, Tae JH. Pancreatic abscess following scrub typhus associated with multiorgan failure. World J Gastroenterol. 2007; 13:3523–3525.
4. Eliashiv A, Olumide F, Norton L, Eiseman B. Depression of cell-mediated immunity in diabetes. Arch Surg. 1978; 113:1180–1183.
5. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007; 23:3–13.
6. Chong VH. Isolated pyogenic pancreatic abscess mimicking a neoplasm. JOP. 2008; 9:309–312.
7. Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002; 97:1386–1391.
8. Seewald S, Groth S, Omar S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos). Gastrointest Endosc. 2005; 62:92–100.
9. Fernández-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998; 228:676–684.
10. Kida M, Itoi T. Current status and future perspective of interventional endoscopic ultrasound in Japan. Dig Endosc. 2009; 21:Suppl 1. S50–S52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download