Abstract
Background/Aims
The invasiveness of a liver biopsy and its inconsistent results have prompted efforts to develop noninvasive tools to evaluate the severity of chronic hepatitis. This study was intended to assess the performance of serum biomarkers for predicting liver fibrosis in patients with chronic viral hepatitis.
Methods
A total of 302 patients with chronic hepatitis B or C, who had undergone liver biopsy, were retrospectively enrolled. We investigated the diagnostic accuracy of several clinical factors for predicting advanced fibrosis (F≥3).
Results
The study population included 227 patients with chronic hepatitis B, 73 patients with chronic hepatitis C, and 2 patients with co-infection (hepatitis B and C). Histological cirrhosis was identified in 16.2% of the study population. The grade of porto-periportal activity was more correlated with the stage of chronic hepatitis compared with that of lobular activity (r=0.640 vs. r=0.171). Fibrosis stage was correlated with platelet count (r=-0.520), aspartate aminotransferase to platelet ratio index (APRI) (r=0.390), prothrombin time (r=0.376), and albumin (r=-0.357). For the diagnosis of advanced fibrosis, platelet count and APRI were the most predictive variables (AUROC=0.752, and 0.713, respectively).
The pathologic examination of the liver provides information regarding the structural integrity as well as the type and degree of injury and fibrosis.1 This information has diagnostic, therapeutic, and prognostic implications. Liver biopsy is currently the gold standard in evaluating the histologic activity in most liver diseases.12 However, liver biopsy has some limitations. First, the invasiveness of the test makes it difficult to for it to be widely applicable, not to mention that it is not welcomed by most patients. The overall rate of serious complications, including bleeding, punctured gall bladder, and pneumothorax was estimated to be 1.1%, using the HALT-C trial data.3 Although the overall complication rate is low, significant hemorrhage is associated with mortality.456 Second, a sampling error may lead to an underdiagnoses of cirrhosis, which occurs in approximately 14.5% of patients.7 Moreover, a single biopsy specimen is limited in its representativeness.89 Lastly, interobserver or intraobserver variability and a small sample size can result in misdiagnosis.71011 These problems have encouraged efforts to develop reliable and noninvasive tools for evaluating the severity of this disease.
Cross-sectional imaging tools, including magnetic resonance imaging, computed tomography, and ultrasound, have been used to assess the features of advanced liver disease and various focal lesions. However, these modalities are insufficient in indicating the early stages of fibrosis. Recently, attempts to develop non-invasive modalities have led to the introduction of ultrasound-based techniques, including transient elastography, acoustic radiation force impulse elastography, and even magnetic resonance elastography.121314 However, these modalities are limited in their utility due to the lack of clinical experience, limited reproducibility, and high expenses.
Liver fibrosis is a dynamic process. However, specimens from liver biopsy represent the disease only at one stage and one time point. Thus, serologic markers can be more reliable in reflecting early changes of stage and injury in lieu of liver biopsy. Moreover, these markers are easy to use, reproducible, and have the ability to be checked repeatedly. Various serologic markers have been evaluated to predict liver fibrosis. However, most previous studies have focused on chronic hepatitis C and lacked validation among other disease populations, which limited their findings to be applicable in clinical practice. This study was intended to assess the performance of serum biomarkers for the prediction of liver fibrosis in patients with chronic viral hepatitis.
This study was conducted at Dankook University Hospital, which is a tertiary medical center in South Korea. We retrospectively analyzed the medicopathologic records of 302 patients who had undergone percutaneous liver biopsy between October 2003 and August 2010. Patients had regular follow ups at the Gastroenterology Internal medicine department. Patients were identified using a search of inpatient and outpatient hospital databases, using in-hospital liver biopsy code and Korean classification of diseases, which is code based on the international classification of diseases diagnosis codes related to hepatitis B or C or liver cirrhosis. The inclusion criteria of liver biopsy were limited to the procedures for the development of treatment plans based on histologic analysis or assessing the prognosis of chronic viral hepatitis and cirrhosis. All procedures for the diagnosis of space-occupying lesions that indicated cancer were excluded. Moreover, etiologies of chronic hepatitis and liver cirrhosis, other than hepatitis B or C virus, were excluded from this study (Fig. 1).
The clinicopathologic relationship between the histologic activity for chronic viral hepatitis and liver fibrosis were evaluated. Pathologic analyses included histologic activities of necroinflammation (grading) and fibrosis (staging). The activities of necroinflammation were analyzed in accordance with separate measurements for lobular activity and porto-periportal activity. To determine whether the markers can be used as a prediction tool for advanced fibrosis (F3, F4), all potential clinicobiochemical variables were assessed against the current gold standard liver biopsy. Patients were also analyzed according to etiology (chronic hepatitis B or chronic hepatitis C) in the subgroup assessment. The protocol of this study was created in accordance with the guidelines of the ethical committee and approved by the institutional review board of Dankook University Hospital (number: 2013-09-012).
All liver biopsies were performed by interventional radiologists under bedside ultrasonic guidance. The specimen was fixed with 10% neutral buffered formalin and embedded in paraffin en bloc. Four-µm-thick microscopic sections were obtained and stained for evaluation with hematoxylin and eosin along with Masson's trichrome. The histologic activity, which indicates the severity of necroinflammation (grading) and the extent of liver fibrosis (staging), was evaluated using the guidelines proposed by the Korean Study Group for the Pathology of Digestive Disease of the Korean Society of Pathologists.1516 The fibrosis score was assessed based on a five-point scale system (F0: no fibrosis, F1: portal fibrosis, F2: periportal fibrosis, F3: septal fibrosis, F4: cirrhosis). The activity was assessed via two categories: porto-periportal activity and lobular activity. The activity score was graded in accordance with the severity of necroinflammation (G0: no necrosis, G1: minimal, G2: mild, G3: moderate, G4: severe). All biopsy specimens were assessed by three specialized pathologists, each with more than 10 years of experience, and a critical review was preformed one more time by one pathologist (L.W) to minimize the uninteded diagnostic errors.
As potential indirect markers of liver fibrosis, laboratory data including complete blood cell count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase, gamma glutamyl transpeptidase, prothrombin time (PT), international normalized ratio, protein, albumin, AST/ALT, and AST-to-platelet ratio index (APRI), which were checked within 1 week before the liver biopsy, were collected. All biochemical variables were evaluated by the in-hospital laboratory medicine department. APRI was calculated in the following manner: the AST level was divided by the upper limit of normal, then, the AST was divided by the platelet count (109/L) and multiplied by 100.17 An AST value of 37 was used as the upper limit of normal; (AST×100) / (37×platelet count). The AST/ALT ratio was calculated from the data collected by the authors. Demographic factors, including age and sex, were also collected. Patients who had incomplete data were excluded from this study.
Based on previous studies of APRI, which is the most widely studied indirect biomarker for predicting fibrosis in chronic hepatitis, a sample size was calculated. According to previous studies of chronic hepatitis C, the area under the receiver-operating characteristic (AUROC) curve of APRI for the prediction of significant fibrosis or cirrhosis ranged from 0.77 to 0.94.1718 We assumed that AUROC would be 0.8 for predicting advanced fibrosis and that the standard deviation would be 0.05. A minimum sample size of 40 was required for each liver cirrhosis and non-cirrhotic hepatitis group. Finally, a total of 302 patients, consisting of 253 non-cirrhotic chronic hepatitis (83.8%) and 49 liver cirrhosis (16.2%) patients, were enrolled.
Continuous variables were analyzed via the student's t-test, and categorical variables were analyzed using Fisher's exact test. The differences in clinicopathologic performance between liver histology and clinicobiochemical variables were assessed using the correlation coefficiency test and the receiver-operating characteristic (ROC) curve, which plots sensitivity over 1-specificity. The correlation coefficiency assessing ordinal variables was analyzed using the Spearman's correlation test, and other variables were assessed using the Pearson's correlation test. AUROC was calculated. A p-value of less than 0.05 was considered to be statistically significant. Analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
In total, 302 patients (198 males [65.6%], 104 females [34.4%]; total mean age 41±13 years [mean±standard deviation]) were retrospectively investigated. Among them, the pathologic diagnoses were as follows: chronic hepatitis B in 227 (41 cirrhotic) patients, chronic hepatitis C in 73 (7 cirrhotic) patients, chronic hepatitis B and C in 2 (1 cirrhotic) patients. The mean AST, ALT and total bilirubin were 101±119 IU/L, 147±175 IU/L and 1.3±5.8 mg/dL, respectively. The mean PT and international normalized ratio were 13.8±11.4 seconds and 1.3±3.1, respectively. The mean platelet count was 164±58 (109/L). The mean albumin was 4.1±2.3 (g/dL). The mean age of patients with chronic hepatitis B vs. chronic hepatitis C was 38±12 years vs. 49±10 years, respectively (p<0.001). The laboratory variables were not different between hepatitis B vs. hepatitis C groups, with the exception of white blood cell (5.38±1.56 vs. 5.77±1.91, p=0.046) (109/L), AST (108±132 vs. 81±63, p=0.043) (IU/L), and ALT (160±192 vs. 109±98, p=0.012) (IU/L). The characteristics of all enrolled patients are summarized in Table 1.
The total distribution of the staging activity was F0: 18 (6.0%), F1: 40 (13.2%), F2: 82 (27.2%), F3: 113 (37.4%), and F4: 49 (16.2%). The total distribution of the porto-periportal activity of grading was G0: 16 (5.3%), G1: 44 (14.6%), G2: 74 (24.5%), G3: 95 (31.5%), G4: 73 (24.2%). The total distribution of the lobular activity of grading was G0: 1 (0.3%), G1: 40 (13.2%), G2: 155 (51.3%), G3: 80 (26.5%), and G4: 26 (8.6%). The severity of necroinflammation (grading) including porto-periportal activity and lobular activity was different between the two groups (p=0.001 and 0.003 for hepatitis B vs. hepatitis C), but the extent of liver fibrosis (staging) was not different. The distribution of the staging and grading of all enrolled patients are summarized in Table 1.
Among the clinical variables, age positively and significantly affected grading (r=0.156, p=0.007) and staging (r=0.421, p<0.001) activity in patients with chronic hepatitis (Fig. 2). Spearman's coefficient of correlation was higher in staging activity than in grading activity.
The correlation between the activity of necroinflammation and liver fibrosis was statistically significant. Among the activities of necroinflammation, porto-periportal activity (r=0.640, p<0.001) revealed a greater significant correlation with fibrotic activity than lobular activity (r=0.171, p=0.003) (Fig. 3).
The other six clinicobiochemical variables were correlated with liver fibrosis. Staging activity was strongly correlated with platelet count (r=-0.520, p<0.001), APRI (r=0.390, p<0.001), PT (r=0.376, p<0.001), albumin (r=-0.357, p<0.001), gamma glutamyl transpeptidase (γ-GT) (r=0.324, p<0.001), and AST/ALT (r=0.302, p<0.001), in descending order (Fig. 4).
In the ROC curve for predicting advanced fibrosis, platelet count (AUROC=0.752) and APRI (AUROC=0.713) were the most valuable predictors, followed by γ-GT (AUROC=0.682), albumin (AUROC=0.673), PT (AUROC=0.660), and AST/ALT (AUROC=0.653), in descending order (Fig. 5).
According to the sub-group analysis, the ROC curve for predicting advanced fibrosis in chronic hepatitis B, platelet count (AUROC=0.778) and APRI (AUROC=0.735) were the most valuable predictors, followed by albumin (AUROC=0.713), γ-GT (AUROC=0.699), AST/ALT (AUROC=0.688), and PT (AUROC=0.678), in descending order (Table 2).
To predict advanced fibrosis in chronic hepatitis C, APRI (AUROC=0.713) was the most valuable predictor, followed by γ-GT (AUROC=0.698), platelet count (AUROC=0.651), PT (AUROC=0.620), AST/ALT (AUROC=0.579), and albumin (AUROC=0.531), in descending order. The AUROC for the prediction of advanced fibrosis in chronic hepatitis B and C are shown in Table 2.
Liver fibrosis is a dynamic process. Hepatic injury via virus, alcohol, bile acid, or fatty acid elicits stellate cell activation along with portal fibroblasts and myofibroblasts in bone marrow.19 A balance between extracellular matrix production and degradation involving matrix metalloproteinase, leads to liver cirrhosis or a reversible recovery state.20 Complications associated with portal hypertension and the development of hepatocellular carcinoma are indicated by the presence of advance hepatic fibrosis. Therefore, the early detection and treatment of liver fibrosis and its complications are important.21 A variety of methods, including serum biomarkers and imaging tools (such as computed tomography, magnetic resonance imaging, ultrasound, transient elastography, and magnetic resonance elastography), have been proposed for the assessment of hepatic fibrosis.121314 Although the results of these methods are encouraging, these techniques are too expensive and time-consuming for clinical implementation.22 Regarding more accurate measurements for the dynamic process of liver fibrosis, serum biomarkers have been evaluated and compared with liver biopsies. These markers are easy to use and are highly reproducible; additionally, they reflect the dynamic change of the liver parenchyme.623 However, similar to the issues encountered in the method of biopsy (including standardization, representativeness and sampling errors), direct markers (measuring extracellular matrix materials, collagenases and their inhibitors, cytokines and chemokines) have some limitations to be considered as reliable markers; however, there are encouraging study results.2324
In this study, the clinicopathologic relationship between histologic activity for chronic viral hepatitis and liver fibrosis was evaluated using indirect markers of liver fibrosis. The major finding of this study was that the platelet count and APRI can be used to reliably predict advanced hepatic fibrosis. Many non-invasive methods for predicting liver fibrosis have been validated in the context of hepatitis C; however, there are a few valuable markers that can be used as indirect markers of liver fibrosis in chronic hepatitis B. Thus, this study enrolled both hepatitis B and C patients. There were more patients with chronic hepatitis B than those with hepatitis C (227 vs. 73). AUROC was 0.752 in platelets and 0.713 in APRI for predicting advanced fibrosis in all patients. These results were concordant in patients with chronic hepatitis B (AUROC of platelets: 0.778, AUROC of APRI: 0.735). A previous study also demonstrated that the AUROC of APRI (F2-4 METAVIR score, ≥3 Ishak score) with chronic hepatitis B was 0.86 in Korean patients.25 Another study also revealed that APRI showed a significant correlation with liver fibrosis of Korean patients with chronic hepatitis B (r=0.501, p<0.001).26 In our study, the platelet count showed a stronger correlation with liver fibrosis (r=-0.520, p<0.001) than APRI. In accordance with a study from China, platelets were the only factor associated with significant fibrosis (F2-4 METAVIR score, ≥3 Ishak score) and cirrhosis (F4 METAVIR score, 5-6 Ishak score) in chronic hepatitis B patients (AUROC=0.63, 0.73, respectively).27
Regarding chronic hepatitis C, a previous meta-analysis with respect to AUROC of the APRI for significant fibrosis and cirrhosis demonstrated values of 0.76 and 0.82, respectively.28 Another meta-analysis demonstrated that the mean AUROC of the APRI for significant fibrosis and cirrhosis were 0.77 and 0.83, respectively.29 The result of our study also showed that AUROC of the APRI for advanced fibrosis in chronic hepatitis C was 0.713. However, the AUROC value of platelet count for predicting advanced fibrosis in chronic hepatitis C was 0.651, which was lower compared with those in chronic hepatitis B, in which the platelet count was the best predictive factor for advanced fibrosis.
The AST/ALT ratio, which is used to differentiate between alcoholic liver disease, cirrhosis, and hepatitis, showed the lowest AUROC for predicting advanced fibrosis (AUROC=0.653) and a correlation coefficiency with fibrosis stage (r=0.320, p<0.001). This result was obtained due to the diversity of stages from hepatitis to cirrhosis in the enrolled patients. Disease distribution could act as a bias (called spectrum bias).30
The overall results in this study are nearly concordant with those of previous studies. However, this study enrolled both chronic hepatitis B and C patients for the integrated evaluation. Another difference was that 75.1% of our patients had chronic hepatitis B. For the prediction of fibrosis, most studies used significant fibrosis (F2-4 METAVIR score, ≥3 Ishak score) as the standard method of analysis. However, this study focused more on severe stages and used advanced fibrosis (F3-4) as the standard method of analysis. This focus could be why AUROCs for predicting advanced fibrosis were slightly lower than those in other studies.
However, only APRI showed a value greater than 0.7 via AUROC (0.735 vs. 0.713 in chronic hepatitis B vs. C), which is commonly irrelevant to the etiology of hepatitis. These results are insufficient, thus inapplicable to clinical practice. There is also a possibility of selection bias. The patients had been followed-up regularly at the tertiary medical center, and the study population had relatively severe chronic liver diseases with high AST and ALT. A total of 80.8% of patients had a staging activity greater than F2; these patients did not show a normal distribution. Moreover, the sample size of this study was small. This spectrum bias makes it difficult to compare the efficiency of biomarkers across different study populations.30
Ideally, a non-invasive biomarker for hepatic fibrosis should be liver-specific, reliable, inexpensive, and useful for monitoring patients over an extended period of time; however, there are no markers that currently satisfy all of these conditions.22 Because the pathophysiology of liver cirrhosis is complex, it is unlikely that a single biomarker will reliably reflect the disease process. Many biomarker panels, including FibroTest, FibroSure, ActiTest, and non-alcoholic fatty liver disease fibrosis score, have been developed to enhance the sensitivity of detecting hepatic fibrosis.313233 However, to date, no panels are widely being used, and this study did not include a panel-form analysis. Moreover, our study was a cross-sectional observational study, and the inclusion criteria did not include alcoholic or non-alcoholic differentiation, which is another etiology of hepatic fibrosis.
There are several limitations to consider when interpreting this present study. Although sample size measurement was done before the initiation of this study, a relatively small number of patients was enrolled and the pre-hypothesized AUROC was not achieved. Moreover, due to its retrospective nature, the results may be limited. The lack of external or internal validation is also a limitation of this study. Another pitfall is the spectrum bias, which may influence the results. Although patients with only viral etiology was included, it is possible that patients with concomitant steatosis may have been included in our study population. Despite these limitations, this study sheds light on the value on non-invasive markers. APRI, in particular, may be a non-invasive tool for predicting advanced hepatic fibrosis not caused by hepatitis B or C. The platelet count and APRI had an increased AUROC in chronic hepatitis B compared with those in chronic hepatitis C. For biomarkers to become the new gold standard in liver biopsy, achieving an appropriate AUROC remains a challenge.34
In conclusion, platelet count and APRI can be considered the most reliable non-invasive tools for predicting liver histologic performance of chronic hepatitis and liver fibrosis. However, until a more definitive conclusion can be made, these biomarkers cannot replace liver biopsy at the moment.
Figures and Tables
 | Fig. 2(A, B) Box plots of age relative to grade and fibrosis stage in patients with chronic hepatitis. The bottom and top edges of the box indicate the interquartile ranges. The lines in the boxes represent the median values. The lines protruding from the box indicate the range of total values. The circles depicted out of the lines indicate the outliers. Age and grade were significantly correlated (r=0.156, p=0.007) (A). Age and fibrosis stage were significantly correlated (r=0.421, p<0.001) (B). |
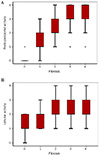 | Fig. 3(A, B) Box plots of activity of necroinflammation relative to fibrosis stage. The bottom and top edges of the box indicate the interquartile ranges. The lines in the boxes represent the median values. The lines protruding from the box indicate the range of total values. The circles depicted out of the lines indicate the outliers. The star depicted out of the lines indicate the extremes. Spearman's coefficient of correlation was higher in porto-periportal activity (r=0.640, p<0.001) (A) than lobular activity (r=0.171, p=0.003) (B) of necroinflammation. |
 | Fig. 4(A-F) Box plots of six clinical variables relative to fibrosis stage. The bottom and top edges of the box indicate the interquartile ranges. The lines in the boxes represent the median values. The lines protruding from the box indicate the range of total values. The circles depicted out of the lines indicate the outliers. The star depicted out of the lines indicate the extremes. Fibrosis stage was well correlated with the platelet count (r=-0.520, p<0.001) (A), APRI (r=0.390, p<0.001) (B), PT (r=0.376, p<0.001) (C), albumin (r=-0.357, p<0.001) (D), γ-GT (r=0.324, p<0.001) (E), and AST/ALT (r=0.302, p<0.001) (F), in order. APRI, aspartate aminotransferase to platelet ratio index; PT, prothrombin time; γ-GT, gamma glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase. |
 | Fig. 5(A-F) ROC curve for predicting advanced fibrosis. Platelet count (AUROC = 0.752) (A) and APRI (AUROC = 0.713) (B) was the most valuable predictors, and the next were γ-GT (AUROC = 0.682) (C), albumin (AUROC = 0.673) (D), PT (AUROC = 0.660) (E), and AST/ALT (AUROC = 0.653) (F), in order. ROC, receiver-operating characteristic; AUROC, area under the receiver-operating characteristic; APRI, aspartate aminotransferase to platelet ratio index; γ-GT, gamma glutamyl transpeptidase; PT, prothrombin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase. |
Table 1
Demographic Data and Clinical Characteristics of Enrolled Patients

Values are presented as mean±standard deviation or n (%) unless otherwise indicated.
WBC, white blood cell; Hb, hemoglobin; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-bil, total bilirubin; ALP, alkaline phosphatase; γ-GT, gamma glutamyl transpeptidase; APRI, AST to platelet ratio index.
References
1. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001; 344:495–500.
2. Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002; 36:5 Suppl 1. S152–S160.
3. Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010; 8:877–883.
4. McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990; 99:1396–1400.
5. Van Thiel DH, Gavaler JS, Wright H, Tzakis A. Liver biopsy. Its safety and complications as seen at a liver transplant center. Transplantation. 1993; 55:1087–1090.
6. Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008; 134:1670–1681.
7. Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002; 97:2614–2618.
8. Maharaj B, Maharaj RJ, Leary WP, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986; 1:523–525.
9. Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003; 38:1449–1457.
10. Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003; 39:239–244.
11. Grønbaek K, Christensen PB, Hamilton‐Dutoit S, et al. Interobserver variation in interpretation of serial liver biopsies from patients with chronic hepatitis C. J Viral Hepat. 2002; 9:443–449.
12. Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008; 134:960–974.
13. Rizzo L, Calvaruso V, Cacopardo B, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011; 106:2112–2120.
14. Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion‐weighted imaging for the staging of hepatic fibrosis: a meta‐analysis. Hepatology. 2012; 56:239–247.
15. Park YN, Chon CY, Park JB, et al. Histological grading and staging of chronic hepatitis standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Korean J Pathol. 1999; 33:337–346.
16. Yu E. Korean Study Group for the Pathology of Digestive Diseases. Histologic grading and staging of chronic hepatitis: on the basis of standardized guideline proposed by the Korean Study Group for the Pathology of Digestive Diseases. Taehan Kan Hakhoe Chi. 2003; 9:42–46.
17. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38:518–526.
18. Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011; 53:726–736.
19. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115:209–218.
20. Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001; 21:373–384.
21. Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004; 99:1160–1174.
22. Castera L. Non-invasive diagnosis of steatosis and fibrosis. Diabetes metab. 2008; 34(6 Pt 2):674–679.
23. Zhou K, Lu LG. Assessment of fibrosis in chronic liver diseases. J Dig Dis. 2009; 10:7–14.
24. Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004; 127:1704–1713.
25. Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008; 40:267–274.
26. Sim SJ, Cheong JY, Cho SW, et al. Efficacy of AST to platelet ratio index in predicting severe hepatic fibrosis and cirrhosis in chronic hepatitis B virus infection. Korean J Gastroenterol. 2005; 45:340–347.
27. Wai CT, Cheng CL, Wee A, et al. Non‐invasive models for predicting histology in patients with chronic hepatitis B. Liver int. 2006; 26:666–672.
28. Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007; 46:912–921.
29. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998; 93:44–48.
30. Guha IN, Myers RP, Patel K, Talwalkar JA. Biomarkers of liver fibrosis: what lies beneath the receiver operating characteristic curve? Hepatology. 2011; 54:1454–1462.
31. Halfon P, Bourliere M, Deydier R, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol. 2006; 101:547–555.
32. Poynard T, Imbert-Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004; 3:8.
33. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007; 45:846–854.
34. Ratziu V. Serum fibrosis markers: death by validation or a leap of faith? J Hepatol. 2010; 53:222–224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download