Abstract
Background/Aims
Peritoneal micrometastasis is known to play an important role in the recurrence of gastric cancer. However, its effects remain equivocal. Herein, we examine the messenger RNA (mRNA) as tumor markers, carcinoembryonic antigen (CEA), and cytokeratin 20 (CK20), in peritoneal washing fluid. Moreover, we evaluate whether these results could predict the recurrence of gastric cancer following curative resection.
Methods
We prospectively enrolled 132 patients with gastric cancers, who had received an operation, between January 2010 and January 2013. The peritoneal lavage fluid was collected at the operation field and semi-quantitative PCR was performed using the primers for CEA and CK20. We excluded patients with stage IA (n=28) early gastric cancer, positive cytologic examination of peritoneal washings (n=7), and those who were lost during follow up (n=18).
Results
A total of 79 patients with gastric cancers were enrolled, and the mean follow-up period was 39.95±19.25 months (range, 5–72 months). According to the multivariate analysis, T4 stage at the initial diagnosis was significantly associated with recurrence. All cases of recurrence were CEA positive and 6 cases were CK20 positive. The positive and negative predictive values of CEA were 32.0% and 100%, respectively, whereas those of CK20 were 37.5% and 71.4%, respectively. Disease free survival of CK20-negative cases was 36.17±20.28 months and that of CK20-positive cases was 32.06±22.95 months (p=0.39).
Go to : 
References
1. Shibata A, Parsonnet J. Stomach cancer. Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3rd ed.New York: Oxford University Press;2006. p. 707–720.

2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

3. Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000; 87:353–357.

4. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.

5. Mori T, Fujiwara Y, Sugita Y, et al. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol. 2004; 11:14–20.

6. de Manzoni G, Verlato G, Di Leo A, et al. Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg. 2006; 30:579–584.

7. Harrison JD, Fielding JW. Prognostic factors for gastric cancer influencing clinical practice. World J Surg. 1995; 19:496–500.

8. Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998; 1:125–133.

9. Roukos DH, Lorenz M, Karakostas K, Paraschou P, Batsis C, Kappas AM. Pathological serosa and node-based classification accurately predicts gastric cancer recurrence risk and outcome, and determines potential and limitation of a Japanese-style extensive surgery for Western patients: a prospective with quality control 10-year follow-up study. Br J Cancer. 2001; 84:1602–1609.

10. Folli S, Morgagni P, Roviello F, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001; 31:495–499.

11. Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg. 2005; 241:247–255.
12. Tímár J, Csuka O, Orosz Z, Jeney A, Kopper L. Molecular pathology of tumor metastasis. II. Molecular staging and differential diagnosis. Pathol Oncol Res. 2002; 8:204–219.
13. Yonemura Y, Fujimura T, Ninomiya I, et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptasepolymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001; 7:1647–1653.
14. Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptasepolymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002; 235:499–506.
15. Osaka H, Yashiro M, Sawada T, Katsuragi K, Hirakawa K. Is a lymph node detected by the dye-guided method a true sentinel node in gastric cancer? Clin Cancer Res. 2004; 10:6912–6918.

16. Matsuda J, Kitagawa Y, Fujii H, et al. Significance of metastasis detected by molecular techniques in sentinel nodes of patients with gastrointestinal cancer. Ann Surg Oncol. 2004; 11(3 Suppl):250S–254S.

17. Yanagita S, Natsugoe S, Uenosono Y, et al. Detection of micro-metastases in sentinel node navigation surgery for gastric cancer. Surg Oncol. 2008; 17:203–210.

18. Katsuragi K, Yashiro M, Sawada T, Osaka H, Ohira M, Hirakawa K. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br J Cancer. 2007; 97:550–556.

19. Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005; 8:142–148.
20. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17:3077–3079.

21. Wu J, Liu X, Cai H, Wang Y. Prediction of tumor recurrence after curative resection in gastric carcinoma based on bcl-2 expression. World J Surg Oncol. 2014; 12:40.

22. Sakakura C, Hagiwara A, Shirasu M, et al. Polymerase chain reaction for detection of carcinoembryonic antigen-expressing tumor cells on milky spots of the greater omentum in gastric cancer patients: a pilot study. Int J Cancer. 2001; 95:286–289.

23. Kodera Y, Nakanishi H, Yamamura Y, et al. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptasepolymerase chain reaction and cytology. Int J Cancer. 1998; 79:429–433.

24. Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg. 2002; 194:568–577.
25. Kunisaki C, Akiyama H, Nomura M, et al. Surgical outcomes in patients with T4 gastric carcinoma. J Am Coll Surg. 2006; 202:223–230.

26. Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996; 23:281–291.
Go to : 
 | Fig. 1.RT-PCR for mRNA of CEA and CK20. RT-PCR, real time polymerase chain reaction; mRNA, messenger RNA; CEA, carcinoembryonic antigen; CK20, cytokeratin 20; GPADH, glyceraldehyde-3-phosphate dehydrogenase. |
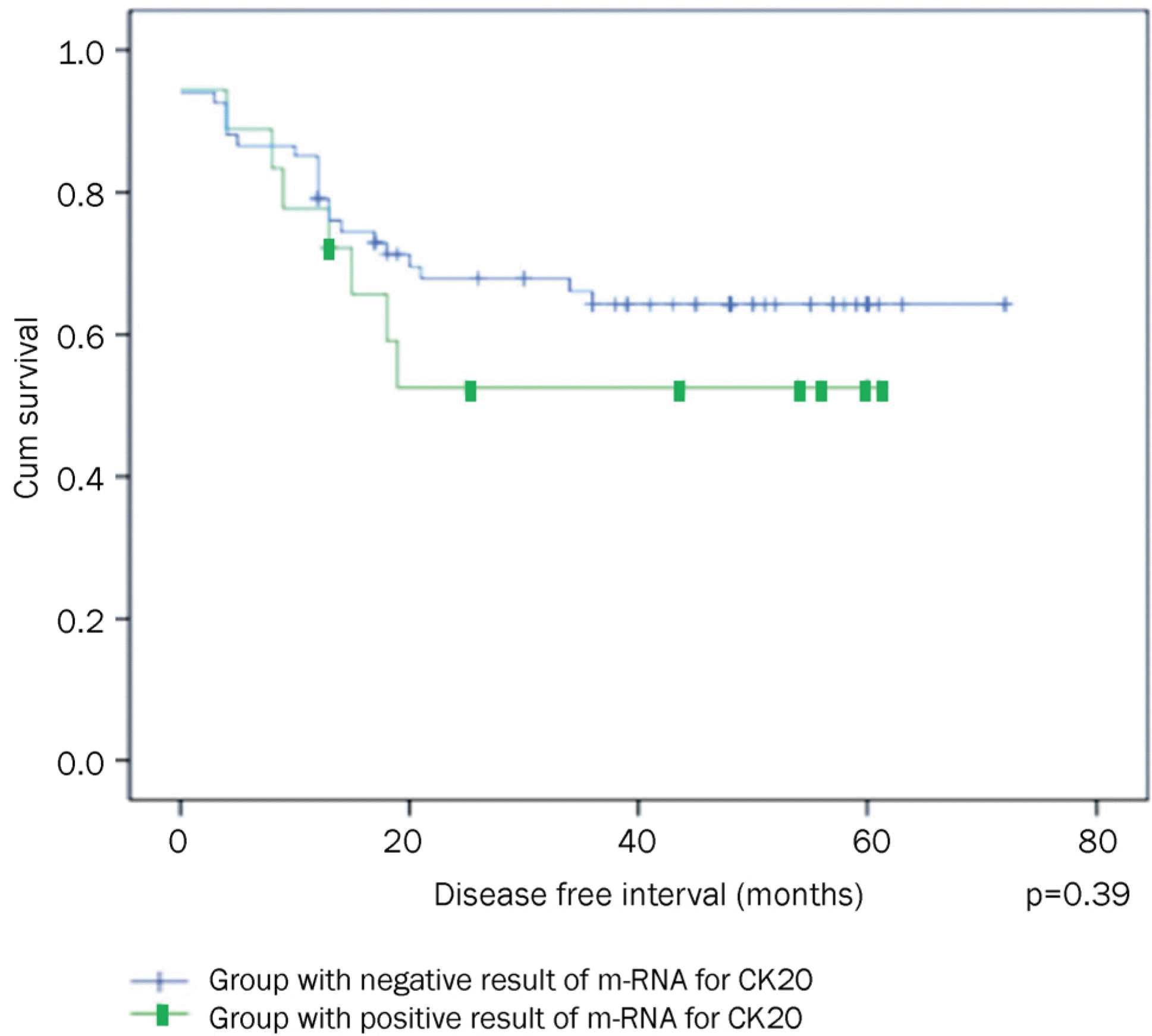 | Fig. 2.Kaplan-Meier curve for analyzing DFS between the CK20 negative and positive groups. DFS, disease free survival; CK20, cytokeratin 20; mRNA, messenger RNA. |
Table 1.
Clinical Features of Recurrence in the Patients with Gastric Cancers
| Recurrence (n=24) | Non-recurrence (n=55) | p-value | |
|---|---|---|---|
| Age | 62.04±13.43 | 62.53±12.08 | 0.91 |
| Sex (male: female) | 16: 8 | 41: 14 | 0.47 |
| Smoke | 7 | 15 | 0.86 |
| Alcohol drinking | 13 | 31 | 0.86 |
| Body mass index (kg/m2) | 23.96±3.16 | 23.01±3.56 | 0.37 |
| Presence of atrophy | 10 | 30 | 0.29 |
| Lauren classification (Diffuse) | 12 | 24 | 0.60 |
| Differentiation (Poorly differentiated / Signet ring cell cancer) | 15 | 27 | 0.08 |
| Lymphatic invasion | 19 | 25 | <0.01 a |
| Vascular invasion | 12 | 10 | <0.01 a |
| Perineural invasion | 15 | 19 | 0.02 a |
| Number of dissected lymph nodes at operation | 42.75±13.84 | 37.15±15.55 | 0.28 |
| T stage at diagnosis (T4) | 18 | 8 | <0.01 a |
| N stage at diagnosis (N3) | 12 | 7 | <0.01 a |
| Chemotherapy | 24 | 42 | − |
| Positive result of mRNA-CEA in peritoneal washing | 24 | 51 | − |
| Positive result of mRNA-CK20 in peritoneal washing | 6 | 10 | 0.49 |
| Recurrence | |||
| Multiple lesions | 10 | ||
| Lymph node | 11 | ||
| Liver | 5 | ||
| Peritoneum | 6 | ||
| Colon | 3 | ||
| Pancreas | 3 | ||
| Bone | 2 | ||
| Ovary | 2 | ||
| Lung | 1 | ||
| Stoma | 2 |
Table 2.
Multivariate Analysis of the Factors of Recurrence in the Patients with Gastric Cancers
| Sig. | Exp(B) | 95.0% C.I. for EXP(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Positive result of mRNA-CK20 in peritoneal washing | 0.22 | 2.69 | 0.56 | 12.88 |
| Differentiation (poorly differentiated and signet ring cell type) | 0.28 | 0.43 | 0.10 | 1.96 |
| Lymphatic invasion | 0.87 | 1.15 | 0.22 | 6.13 |
| Vascular invasion | 0.27 | 2.51 | 0.48 | 13.02 |
| Perineural invasion | 0.81 | 1.19 | 0.29 | 4.96 |
| T stage at diagnosis (T4) | <0.01 a | 11.79 | 2.23 | 62.21 |
| N stage at diagnosis (N3) | 0.17 | 2.91 | 0.64 | 13.30 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download