Abstract
Background/Aims
Mesenchymal stem cells (MSCs) are multipotent progenitor cells currently under investigation for its efficacy as the treatment for inflammatory bowel disease. In this study, we evaluated the efficacy of tonsil-derived mesenchymal stem cells (T-MSCs) as a novel source of mesenchymal stem cells and traced their localization in a murine model of acute colitis induced by dextran sulfate sodium (DSS).
Methods
C57BL/6 mice were randomly assigned to the following three groups: the normal control group, DSS colitis group (DSS+phosphate buffered saline), and T-MSC group (DSS+T-MSCs, 1106). The severity of colitis was assessed by determining the severity of symptoms of colitis, colon length, histopathologic grade, and levels of inflammatory cytokines. T-MSCs labeled with PKH26 were traced in vivo.
Results
The T-MSC group, compared with the DSS colitis group, showed a significantly lower disease activity index (11.3±1.5 vs. 8.3±1.9, p=0.015) at sacrifice and less reduction of body weight (−17.1±5.0% vs. −8.1±6.9%, p=0.049). In the T-MSC group, the histologic colitis score was significantly decreased compared with the DSS colitis group (22.6±3.8 vs. 17.0±3.4, p=0.039). IL-6 and IL-1β, the pro-inflammatory cytokines, were also significantly reduced after a treatment with T-MSCs. In vivo tracking revealed no PKH26-la-belled T-MSCs in the colonic tissue of mice with acute colitis.
Go to : 
References
1. Nagaishi K, Arimura Y, Fujimiya M. Stem cell therapy for inflammatory bowel disease. J Gastroenterol. 2015; 50:280–286.

2. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

3. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007; 117:514–521.

4. Miehsler W, Novacek G, Wenzl H, et al. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010; 4:221–256.

5. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749.

6. Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005; 11:1198–1211.
7. Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. 2012; 1:200–205.

8. Chen QQ, Yan L, Wang CZ, et al. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013; 19:4702–4717.

9. Kim HS, Shin TH, Lee BC, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013; 145:1392–1403. e1–8.

10. Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009; 58:929–939.

11. Cho KA, Kim JY, Kim HS, Ryu KH, Woo SY. Tonsil-derived mesenchymal progenitor cells acquire a follicular dendritic cell phenotype under cytokine stimulation. Cytokine. 2012; 59:211–214.

13. Janjanin S, Djouad F, Shanti RM, et al. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther. 2008; 10:R83.

14. Ryu KH, Cho KA, Park HS, et al. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy. 2012; 14:1193–1202.

15. Ryu KH, Kim SY, Kim YR, et al. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp Cell Res. 2014; 326:143–154.

16. Stevceva L, Pavli P, Husband A, Ramsay A, Doe WF. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun. 2001; 2:309–316.

17. Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003; 52:713–719.

18. Liang J, Zhang H, Wang D, et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012; 61:468–469.

19. Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA Jr. Mesenchymal stem cell therapy for inflammatory bowel disease: a systematic review and metaanalysis. Inflamm Bowel Dis. 2015; 21:2696–2707.
20. Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2015; 149:918–927.e6.

21. Park YS, Kim HS, Jin YM, et al. Differentiated tonsil-derived mesenchymal stem cells embedded in Matrigel restore parathyroid cell functions in rats with parathyroidectomy. Biomaterials. 2015; 65:140–152.

22. Park S, Choi Y, Jung N, et al. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med. 2016; 37:1209–1220.

23. Park M, Kim YH, Woo SY, et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci Rep. 2015; 5:8616.

24. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533.

25. Seegert D, Rosenstiel P, Pfahler H, Pfefferkorn P, Nikolaus S, Schreiber S. Increased expression of IL-16 in inflammatory bowel disease. Gut. 2001; 48:326–332.

26. Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005; 337:647–654.
27. Ito H. Treatment of Crohn's disease with anti-IL-6 receptor antibody. J Gastroenterol. 2005; 40(Suppl 16):32–34.

28. Arai Y, Takanashi H, Kitagawa H, Okayasu I. Involvement of inter-leukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine. 1998; 10:890–896.

29. Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGFbeta. J Neuroimmunol. 2009; 207:83–91.
30. He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012; 57:3136–3144.

31. Park M, Kim YH, Ryu JH, Woo SY, Ryu KH. Immune suppressive effects of tonsil-derived mesenchymal stem cells on mouse bone-marrow-derived dendritic cells. Stem Cells Int. 2015; 2015:106540.

32. Taylor BS, de Vera ME, Ganster RW, et al. Multiple NF-kappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998; 273:15148–15156.
33. Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106:1755–1761.

34. Castelo-Branco MT, Soares ID, Lopes DV, et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012; 7:e33360.

35. Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013; 41:283–287.

36. Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012; 27:3037–3042.

Go to : 
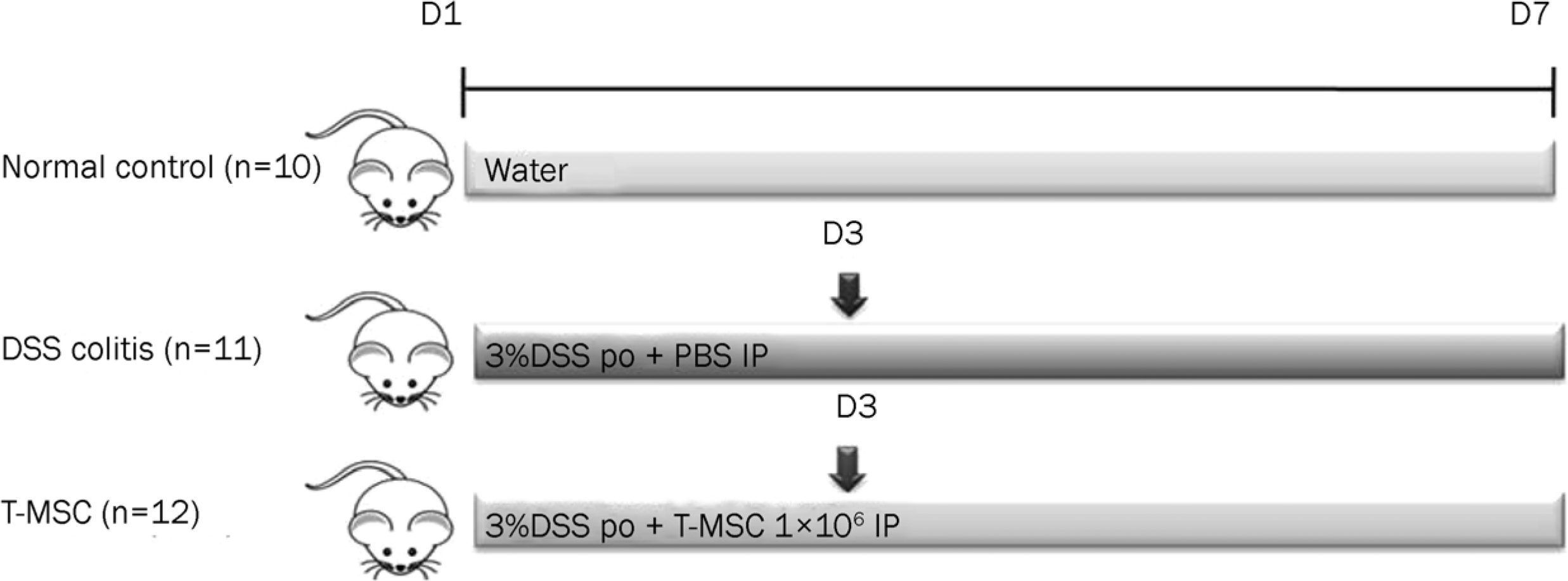 | Fig. 1.Induction of acute colitis. Acute colitis was induced by 3% DSS treatment for 7 days. T-MSC was injected intraperitoneally on day 3 after an induction of acute colitis. PBS only was administered as a control in the DSS colitis group. DSS, dextran sulfate sodium; T-MSC, tonsil-derived mesenchymal stem cell; PBS, phosphate-buffered salilne. |
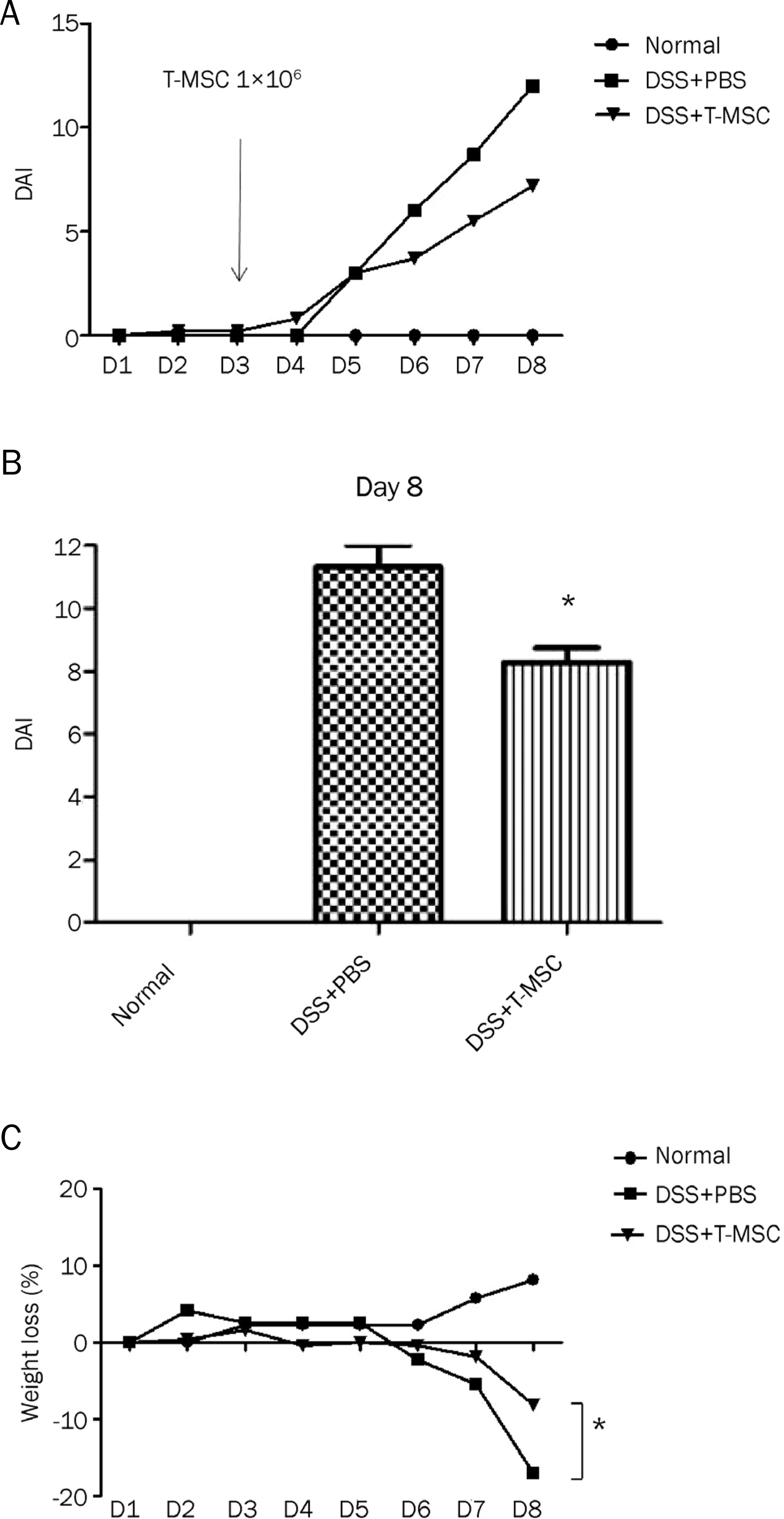 | Fig. 2.Effect of treatment with T-MSCs on inflammatory symptoms. T-MSC ameliorated the inflammatory symptoms assessed by the DAI in DSS-induced acute colitis. (A) The difference of DAI between the T-MSC group and the DSS colitis group assessed daily was maximized by day 8. (B) The mean DAI of the T-MSC group on day 8 was significantly lower than that of the DSS colitis group (11.3±1.5 vs. 8.3±1.9, p=0.015). (C) The reduction of body weight at sacrifice was attenuated with T-MSCs treatment (−17.1±5.0% vs. −8.1±6.9%, p=0.049). Data are presented as the mean±SEM (*p<0.05). T-MSCs, tonsil-derived mesenchymal stem cells; DAI, disease activity index; DSS, dextran sulfate sodium; PBS, phosphate-buffered salilne. |
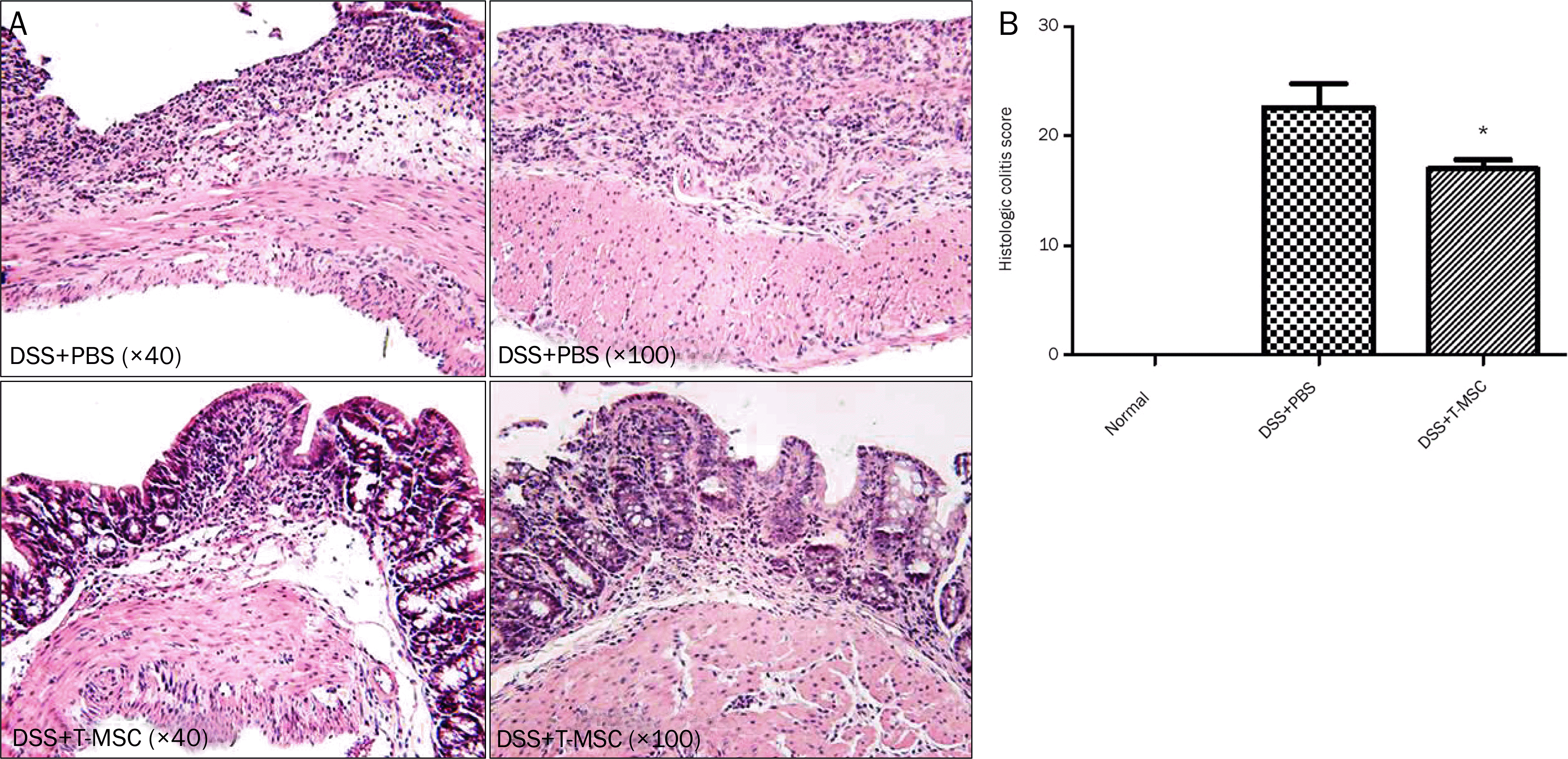 | Fig. 3.Treatment with T-MSCs reduced the colonic damage microscopically. (A) The induction of acute colitis showed an extensive infiltration of inflammatory cells, crypt damage, edema, and ulceration. Treatment of T-MSCs reduced the infiltration of inflammatory cells and extent of disease. (B) The histologic colitis score was significantly reduced after T-MSCs treatment (* p<0.05). DSS, dextran sulfate sodium; PBS, phosphate-buffered sal-ilne; T-MSC, tonsil-derived mesenchymal stem cell. |
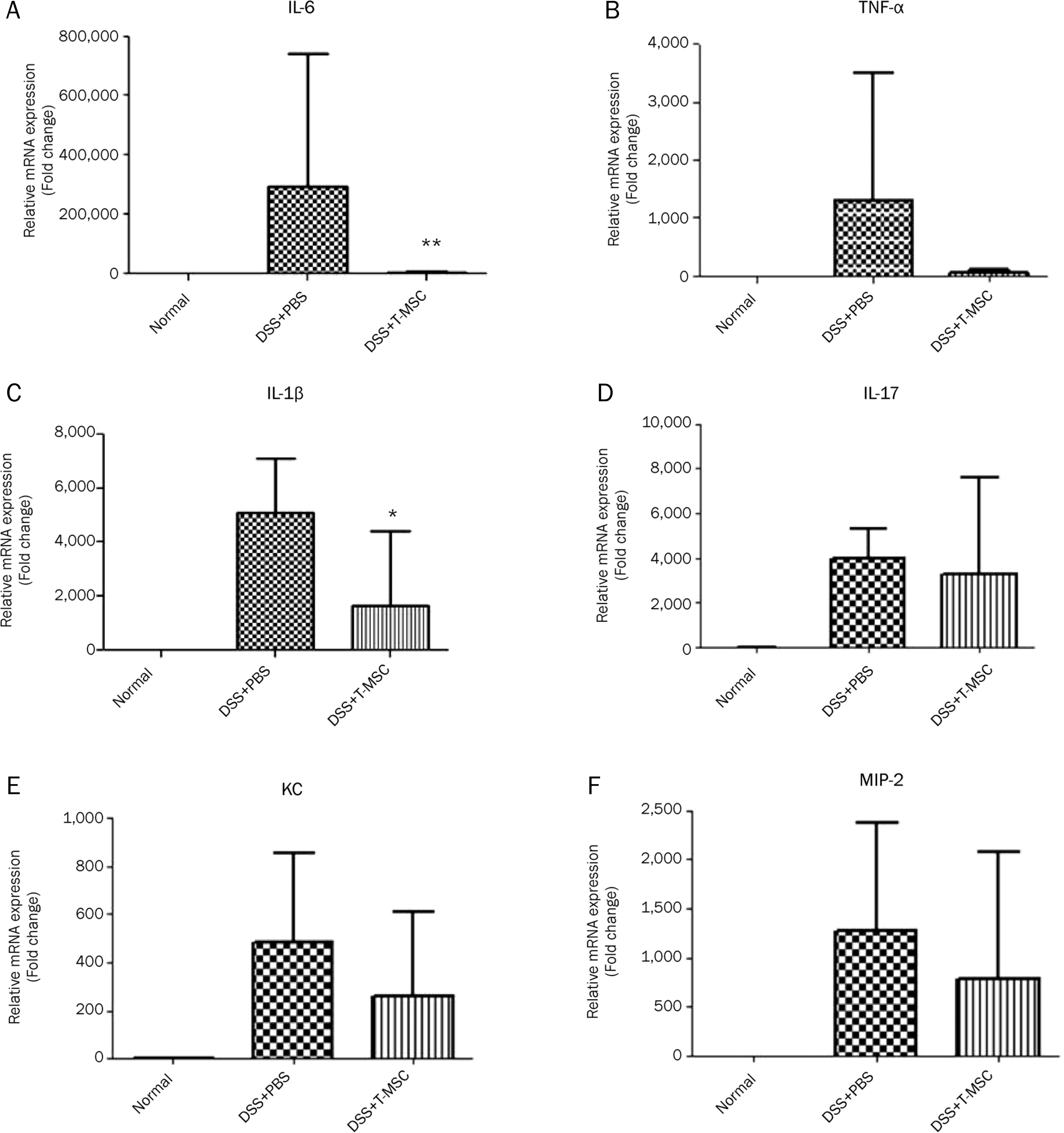 | Fig. 4.The effect of T-MSCs on the expression of pro-inflammatory cytokines, IL-6, TNF-α, IL-1β and IL-17 and chemokines, KC, MIP-2. The mRNA expression of inflammatory mediators was measured in the colonic mucosa by real-time PCR. The level of IL-6 (A) and IL-1β (C) was reduced significantly in the colonic mucosa after a treatment with T-MSCs compared with the DSS colitis group. The level of TNF-α (B), IL-17 (D), KC (E), and MIP-2 (F) were not different between the two groups. Data are presented as the mean±SEM. (*p<0.05, **p<0.01). T-MSCs, tonsil-derived mesenchymal stem cells. |
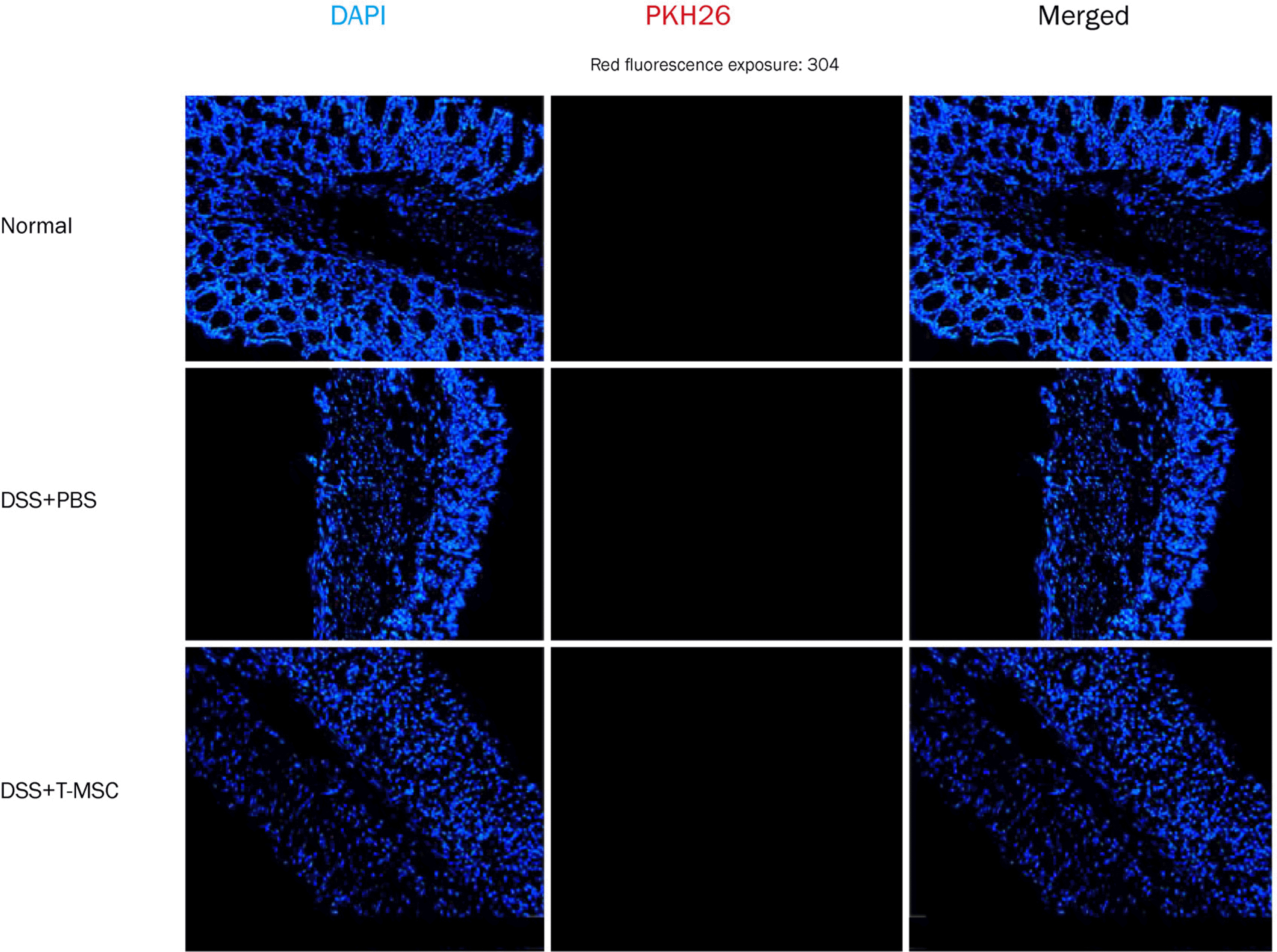 | Fig. 5.Fluorescent microscopy analysis of T-MSC labeled with PKH26. The localization was measured 5 days after intraperitoneal injection. Microscopic examination revealed no distribution of red colored T-MSC-PKH26 cells in the colon of mice with acute colitis. In merge images, nuclei were stained with DAPI (blue). Original magnificationx200. T-MSC, tonsil-derived mesenchymal stem cell; DSS, dextran sulfate sodium; PBS, phosphate-buffered salilne. |
Table 1.
Primer Sequences of the Reverse Transcription Polymerase Chain Reaction (real time-PCR)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download