Abstract
Background/Aims
Optimized regimen has not yet been established for failures of multiple Helicobacter pylori (H. pylori) eradication. Hence, we aimed to evaluate the efficacy of rifabutin-based rescue therapy, at least after three eradication failures.
Methods
Twelve patients, who failed in the treatment for H. pylori eradication at least three times, were consecutively enrolled between 2007 and 2015 at Seoul National University Bundang Hospital. The rifabutin-based rescue regimen was consisted of proton pump inhibitor (PPI), rifabutin (150 mg b.i.d.), and amoxicillin (1 g b.i.d.), given for 7 or 14 days. MIC concentration test by the agar dilution method was performed on six patients prior to rifabutin-based rescue therapy.
Results
One patient did not take this regimen, and per-protocol (PP) analysis was performed in 11 patients. The overall eradication rate by intention-to-treat and PP analysis with rifabutin-based rescue therapy was 50.0% (6/12 patients) and 54.5% (6/11 patients), respectively. There was no difference of the eradication rate depending on the underlying disease, smoking, alcohol, number of previous eradication failures, and CYP2C19 genotype. All of the six patients were susceptible to rifabutin, but only three of them suc-ceeded in eradicating with H. pylori. Side effe cts occurred in two patients (18.2%), and compliance was 90.9%.
Conclusions
Even the eradication rate of rifabutin-based rescue therapy was not very good. Rifabutin-based rescue therapy could be considered as a rescue therapy, perhaps as the fourth or the fifth-line treatment option. No correlation of rifabutin sensitivity with eradication success rate of H. pylori suggests that frequent administration of high dose PPI and amoxicillin might be important.
Go to : 
References
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–1315.

2. Bartnik W. Clinical aspects of Helicobacter pylori infection. Pol Arch Med Wewn. 2008; 118:426–430.
3. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002; 347:1175–1186.
4. Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther. 2002; 16:1047–1057.
5. Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004; 10:668–671.
6. Bigard MA, Delchier JC, Riachi G, Thibault P, Barthelemy P. One-week triple therapy using omeprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment Pharmacol Ther. 1998; 12:383–388.
7. Lee JY, Kim N, Kim MS, et al. Factors affecting first-line triple therapy of H. pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014; 59:1235–1243.
8. Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.
9. Gisbert JP, Pajares JM. Helicobacter pylori "rescue" therapy after failure of two eradication treatments. Helicobacter. 2005; 10:363–372.
10. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007; 56:772–781.
11. Lim HC, Lee YJ, An B, Lee SW, Lee YC, Moon BS. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter. 2014; 19:455–461.
12. Heep M, Beck D, Bayerdorffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999; 43:1497–1499.
13. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012; 35:209–221.
14. Kunin CM. Antimicrobial activity of rifabutin. Clin Infect Dis. 1996; 22(Suppl 1):S3–S13. discussion S13-S14.

15. Gisbert JP, Castro-Fernandez M, Perez-Aisa A, et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012; 35:941–947.
16. Akada JK, Shirai M, Fujii K, Okita K, Nakazawa T. In vitro anti-Helicobacter pylori activities of new rifamycin derivatives, KRM-1648 and KRM-1657. Antimicrob Agents Chemother. 1999; 43:1072–1076.
17. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011; 17:3971–3975.
18. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006; 28:6–13.
19. Lee JW, Kim N, Kim JM, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013; 18:206–214.
20. De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010; 19:409–414.
21. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013; 62:34–42.
22. Hwang TJ, Kim N, Kim HB, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010; 44:536–543.
23. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy–the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999; 13:1047–1055.
24. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012; 61:646–664.
25. Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009; 24:1587–1600.
26. Savarino V, Zentilin P, Pivari M, et al. The impact of antibiotic resistance on the efficacy of three 7-day regimens against Helicobacter pylori. Aliment Pharmacol Ther. 2000; 14:893–900.
27. Gisbert JP, Castro-Fernandez M, Bermejo F, et al. Third-line rescue therapy with levofloxacin after two H. pylori treatment failures. Am J Gastroenterol. 2006; 101:243–247.

28. Perri F, Festa V, Clemente R, et al. Randomized study of two "rescue" therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am J Gastroenterol. 2001; 96:58–62.
29. Mori H, Suzuki H, Matsuzaki J, et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: A pilot study. United European Gastroenterol J. 2016; 4:380–387.
30. Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifa-butin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2006; 23:481–488.
31. MacGowan AP, Bowker KE. Continuous infusion of beta-lactam antibiotics. Clin Pharmacokinet. 1998; 35:391–402.
32. Midolo PD, Turnidge JD, Munckhof WJ. Is bactericidal activity of amoxicillin against Helicobacter pylori concentration dependent? Antimicrob Agents Chemother. 1996; 40:1327–1328.
33. Athamna A, Athamna M, Medlej B, Bast DJ, Rubinstein E. In vitro post-antibiotic effect of fluoroquinolones, macrolides, beta-lac-tams, tetracyclines, vancomycin, clindamycin, linezolid, chlor-amphenicol, quinupristin/dalfopristin and rifampicin on Bacillus anthracis. J Antimicrob Chemother. 2004; 53:609–615.
34. Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012; 36:972–979.
35. Sjostedt S, Sagar M, Lindberg G, Wikstrom B, Nord CE, Seensalu R. Prolonged and profound acid inhibition is crucial in Helicobacter pylori treatment with a proton pump inhibitor combined with amoxicillin. Scand J Gastroenterol. 1998; 33:39–43.
36. Sugimoto M, Shirai N, Nishino M, et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment Pharmacol Ther. 2012; 36:627–634.
37. Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015; 13:895–905.e5.
38. Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007; 63:743–749.
39. Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001; 69:158–168.
40. Kang JM, Kim N, Lee DH, et al. Effect of the CYP2C19 polymorphism on the eradication rate of Helicobacter pylori infection by 7-day triple therapy with regular proton pump inhibitor dosage. J Gastroenterol Hepatol. 2008; 23(8 Pt 1):1287–1291.
41. Zhao F, Wang J, Yang Y, et al. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor-based triple therapy for Helicobacter pylori eradication: a metaanalysis. Helicobacter. 2008; 13:532–541.
42. Robinson M. Review article: the pharmacodynamics and pharmacokinetics of proton pump inhibitors–overview and clinical implications. Aliment Pharmacol Ther. 2004; 20(Suppl 6):1–10.
43. Kuo CH, Lu CY, Shih HY, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014; 20:16029–16036.
44. McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012; 36:414–425.
45. Hokari K, Sugiyama T, Kato M, et al. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection and CYP2C19 genetic polymorphism. Aliment Pharmacol Ther. 2001; 15:1479–1484.
46. Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004; 76:290–301.

47. Griffith DE, Brown BA, Girard WM, Wallace RJ Jr. Adverse events associated with high-dose rifabutin in macrolide-containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 1995; 21:594–598.

48. Apseloff G, Fluids G, LaBoy-Goral L, Kraut E, Vincent J. Severe neutropenia caused by recommended prophylactic doses of rifabutin. Lancet. 1996; 348:685.

49. Bock H, Koop H, Lehn N, Heep M. Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: preliminary experience. J Clin Gastroenterol. 2000; 31:222–225.
50. Suzuki S, Suzuki H, Nishizawa T, et al. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion. 2009; 79:1–4.
Go to : 
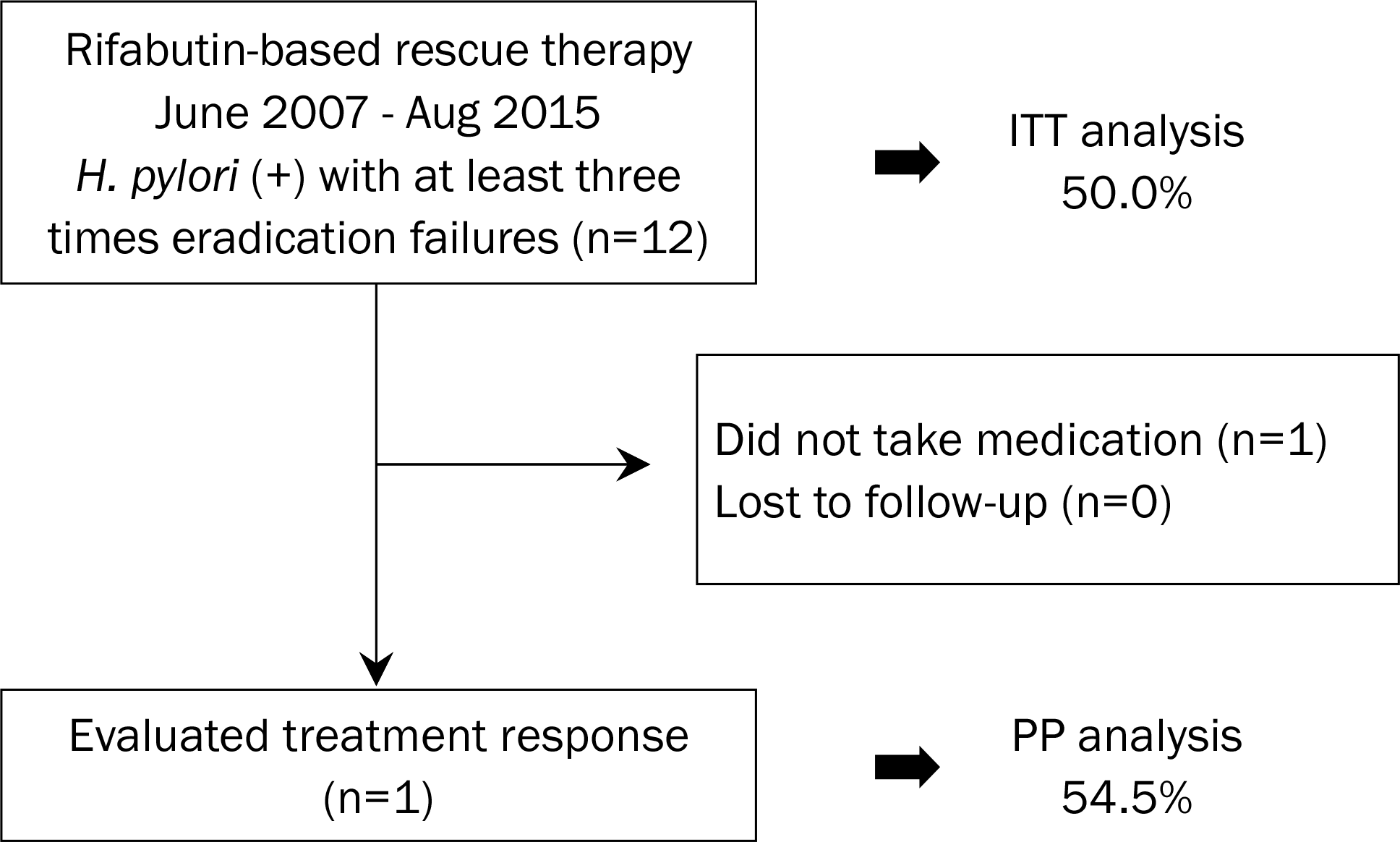 | Fig. 1.Flow chart of the study and eradication rate by rifabutin-based rescue therapy. ITT, intention-to-treat; PP, per-protocol. |
Table 1.
Comparison of Eradication Group and Non-eradication Group
| Variable | Total (n=11) | Eradication group (n=6) | Non-eradication group (n=5) | p-value |
|---|---|---|---|---|
| Age (yr) | 53.9±8.53 | 53.8±10.09 | 54.0±7.4 | 0.976 a |
| Male/female | 10/1 | 6/0 | 4/1 | 0.251 b |
| Underlying disease | 0.325 b | |||
| Benign gastric ulcer | 3 (27.3) | 1 (16.7) | 2 (40) | |
| Duodenal ulcer | 2 (18.2) | 2 (33.3) | 0 | |
| Post ESD d/t EGC | 1 (9.1) | 1 (16.7) | 0 | |
| Non-ulcer dyspepsia | 5 (45.5) | 2 (33.3) | 3 (60) | |
| Smoking | 0.535 b | |||
| Never | 4 (36.4) | 2 (33.3) | 2 (40.0) | |
| Ex-smoker | 3 (27.3) | 1 (16.7) | 2 (40.0) | |
| Current | 4 (36.4) | 3 (50.0) | 1 (20.0) | |
| Alcohol | 0.323 b | |||
| None | 3 (27.3) | 1 (16.7) | 2 (40.0) | |
| Past | 2 (18.2) | 2 (33.3) | 0 | |
| Current | 6 (54.5) | 3 (50.0) | 3 (60.0) | |
| Eradication failure number | 0.497 b | |||
| 3 | 7 (63.6) | 3 (50.0) | 4 (80.0) | |
| 4 | 3 (27.3) | 2 (33.3) | 1 (20.0) | |
| 5 | 1 (9.1) | 1 (16.7) | 0 | |
| Test of confirm H. pylori infection before rifabutin | 0.064 b | |||
| 13C-urea breath test | 8 (72.7) | 3 (50.0) | 5 (100.0) | |
| Culture, Histology and CLOtest | 3 (27.3) | 3 (50.0) | 0 | |
| Duration of treatment (day) | 0.122 b | |||
| 7 | 5 (45.5) | 4 (66.7) | 1 (20.0) | |
| 14 | 6 (54.5) | 2 (33.3) | 4 (80.0) | |
| Proton pump inhibitor dose | 0.371 b | |||
| Lansoprazole 15 mg b.i.d. | 5 (45.5) | 3 (50.0) | 2 (40.0) | |
| Lansoprazole 30 mg q.d. | 1 (9.1) | 0 | 1 (20.0) | |
| Lansoprazole 30 mg b.i.d. | 4 (36.4) | 3 (50.0) | 1 (20.0) | |
| Esomeprazole 40 mg b.i.d. | 1 (9.1) | 0 | 1 (20.0) | |
| CYP2C19 genotype(n=8) | 0.465 b | |||
| HomEM | 4 (50.0) | 1 (33.3) | 3 (60.0) | |
| HetEM | 4 (50.0) | 2 (66.7) | 2 (40.0) | |
| Test for eradication confirmation | 0.887 b | |||
| 13 C-urea breath test | 9 (81.8) | 5 (83.3) | 4 (80.0) | |
| Culture, Histology and CLOtest | 2 (18.2) | 1 (16.7) | 1 (20.0) |
Table 2.
Baseline Characteristics of 11 Patients
Table 3.
Minimal Inhibitory Concentration of Helicobacter pylori
| Patient number | Sex/Age | Antibiotics (μ g/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AMO (R: ≥0.5) | CLA (R: >1) | MTZ (R: >8) | TC (R: >4) | CPR (R: >1) | Rifa (R: ≥0.25) | LEVO (R: >1) | MOXI (R: >1) | ||
| 1 | M/45 | ≤0.125 | 2–4 a | 16–32 a | ≤1 | ≤0.015 | ≤0.015 | 4–8 a | 8–16 a |
| 3 | M/47 | 1–2 a | 4–8 a | ≥32 a | ≤1 | 2–4 a | ≤0.015 | 4–8 a | 4–8 a |
| 6 | M/71 | ≤0.125 | 2–4 a | 16–32 a | ≤1 | 2–4 a | ≤0.015 | ≥2 a | ≥2 a |
| 7 | M/45 | ≤0.125 | 2–4 a | ≤2 | ≤1 | ≤0.25 | ≤0.015 | ≤0.125 | ≤0.125 |
| 8 | F/48 | 0.125–0.25 | ≥32 a | 16–32 a | 8∼16 a | 0.06–0.125 | 0.06–0.125 | 4–8 a | 8–16 a |
| 9 | M/56 | ≤0.125 | ≥32 a | ≥32 a | ≤1 | ≤0.06 | ≤0.015 | 8–16 a | 8–16 a |
Resistant cut-off values were defined as >0.5 μ g/mL for AMO, >1 μ g/mL for CLA, >8 μ g/mL for MTZ, >4 μ g/mL for TC, >1 μ g/mL for CPR, LEVO and MOXI, ≥0.25 μ g/m for Rifa.
Table 4.
Adverse Events and Compliance of the Subjects
| Variable | Eradication group (n=6) | Non-eradication group (n=5) | p-value |
|---|---|---|---|
| Bloating | 0 | 0 | |
| Epigastric soreness | 1 | 0 | |
| Anorexia | 0 | 0 | |
| Taste distortions | 0 | 1 | |
| Nausea | 0 | 0 | |
| Vomiting | 0 | 0 | |
| Abdominal pain | 0 | 1 | |
| Headache | 0 | 0 | |
| Dyspepsia | 0 | 1 | |
| Diarrhea | 0 | 1 | |
| Constipation | 0 | 0 | |
| Reflux | 0 | 1 | |
| Total patient | 1 (16.7) | 1 (20) a | 1.00 b |
| Major c | 1 | 0 | 0.368 b |
| Minor | 0 | 1 | |
| Total event | 1 | 5a | |
| Compliance | 5 (83.3) | 5 (100) | 0.389 b |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download