Abstract
Acute kidney injury (AKI) is one of the most common manifestations encountered in clinical practice. It is associated with high morbidity and mortality in cirrhotic pre- and post-transplantation patients. Hepatorenal syndrome (HRS), a special form of AKI in cirrhotic patients, was recognized as a consequence of renal vasoconstriction from systemic/renal hemodynamic alterations developed in advanced cirrhosis with portal hypertension. Recently, multiple factors— such as infection/inflammation, underlying glomerulonephritis, bile cast, or increased abdominal pressure— have been considered to contribute to renal dysfunction in cirrhotic patients, which were presumed to induce HRS. Moreover, in addition to changing the definition of AKI in the nephrologic guidelines, the new AKI definition for early diagnosis and intervention based on characteristics of liver cirrhosis has been proposed in an international meeting. This article provides a comprehensive and recent review of AKI definition, laying out the topics in accordance with the pathophysiologic mechanisms and therapeutic interventions of AKI in cirrhotic patients with portal hypertension.
Go to : 
References
1. Wu CC, Yeung LK, Tsai WS, et al. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006; 65:28–33.

2. Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008; 48:2064–2077.

3. Hampel H, Bynum GD, Zamora E, El-Serag HB. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001; 96:2206–2210.

4. Terra C, Guevara M, Torre A, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005; 129:1944–1953.

5. Cholongitas E, Senzolo M, Patch D, Shaw S, O'Beirne J, Burroughs AK. Cirrhotics admitted to intensive care unit: the impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol. 2009; 21:744–750.

6. Weismüller TJ, Negm A, Becker T, et al. The introduction of MELD- based organ allocation impacts 3-month survival after liver transplantation by influencing pretransplant patient characteristics. Transpl Int. 2009; 22:970–978.
7. Cárdenas A. Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol. 2005; 100:460–467.
8. Baraldi O, Valentini C, Donati G, et al. Hepatorenal syndrome: Update on diagnosis and treatment. World J Nephrol. 2015; 4:511–520.

9. Thabut D, Massard J, Gangloff A, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007; 46:1872–1882.

10. van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013; 84:192–197.

11. Trawalé JM, Paradis V, Rautou PE, et al. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int. 2010; 30:725–732.

12. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–R212.
13. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31.

14. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012; 2:1–138.
15. Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015; 62:968–974.

16. Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007; 35:1837–1843.

17. Jenq CC, Tsai MH, Tian YC, et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med. 2007; 33:1921–1930.

18. Tinti F, Umbro I, Meçule A, et al. RIFLE criteria and hepatic function in the assessment of acute renal failure in liver transplantation. Transplant Proc. 2010; 42:1233–1236.

19. Valette X, du Cheyron D. A critical appraisal of the accuracy of the RIFLE and AKIN classifications in defining "acute kidney in-sufficiency" in critically ill patients. J Crit Care. 2013; 28:116–125.

20. Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013; 59:482–489.

21. Karapanagiotou A, Dimitriadis C, Papadopoulos S, et al. Comparison of RIFLE and AKIN criteria in the evaluation of the frequency of acute kidney injury in post-liver transplantation patients. Transplant Proc. 2014; 46:3222–3227.

22. Fujii T, Uchino S, Takinami M, Bellomo R. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol. 2014; 9:848–854.

23. Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X. Beijing Acute Kidney Injury Trial (BAKIT) workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014; 18:R144.

24. Pan HC, Chien YS, Jenq CC, et al. Acute kidney injury classification for critically ill cirrhotic patients: a comparison of the KDIGO, AKIN, and RIFLE classifications. Sci Rep. 2016; 6:23022.

25. Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988; 39:465–490.

26. Cholongitas E, Shusang V, Marelli L, et al. Review article: renal function assessment in cirrhosis: difficulties and alternative measurements. Aliment Pharmacol Ther. 2007; 26:969–978.
27. Dimeski G, McWhinney B, Jones B, Mason R, Carter A. Extent of bilirubin interference with Beckman creatinine methods. Ann Clin Biochem. 2008; 45:91–92.

28. Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007; 56:1310–1318.

29. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(2 Suppl 1):S1–S266.
30. Wong F, Nadim MK, Kellum JA, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011; 60:702–709.

31. Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005; 42:439–447.

32. Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008; 28:81–95.

33. Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010; 51:219–226.

34. Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology. 2016; 63:983–992.

35. Grangé JD, Amiot X. Nitric oxide and renal function in cirrhotic patients with ascites: from physiopathology to practice. Eur J Gastroenterol Hepatol. 2004; 16:567–570.
36. Shah N, Mohamed FE, Jover-Cobos M, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013; 33:398–409.

37. Shah N, Dhar D, El Zahraa Mohammed F, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012; 56:1047–1053.

38. Gupta A, Quigg RJ. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis. 2015; 22:343–351.

39. Sumida K, Ubara Y, Hoshino J, et al. Hepatitis C virus-related kidney disease: various histological patterns. Clin Nephrol. 2010; 74:446–456.
40. Tissandié E, Morelle W, Berthelot L, et al. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int. 2011; 80:1352–1363.

41. Umgelter A, Reindl W, Wagner KS, et al. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care. 2008; 12:R4.

42. Chang Y, Qi X, Li Z, et al. Hepatorenal syndrome: insights into the mechanisms of intraabdominal hypertension. Int J Clin Exp Pathol. 2013; 6:2523–2528.
43. Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005; 68:362–370.

44. Sen S, Davies NA, Mookerjee RP, et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004; 10:1109–1119.

45. Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002; 36:949–958.

46. Schmidt LE, Wang LP, Hansen BA, Larsen FS. Systemic hemodynamic effects of treatment with the molecular adsorbents recirculating system in patients with hyperacute liver failure: a prospective controlled trial. Liver Transpl. 2003; 9:290–297.

Go to : 
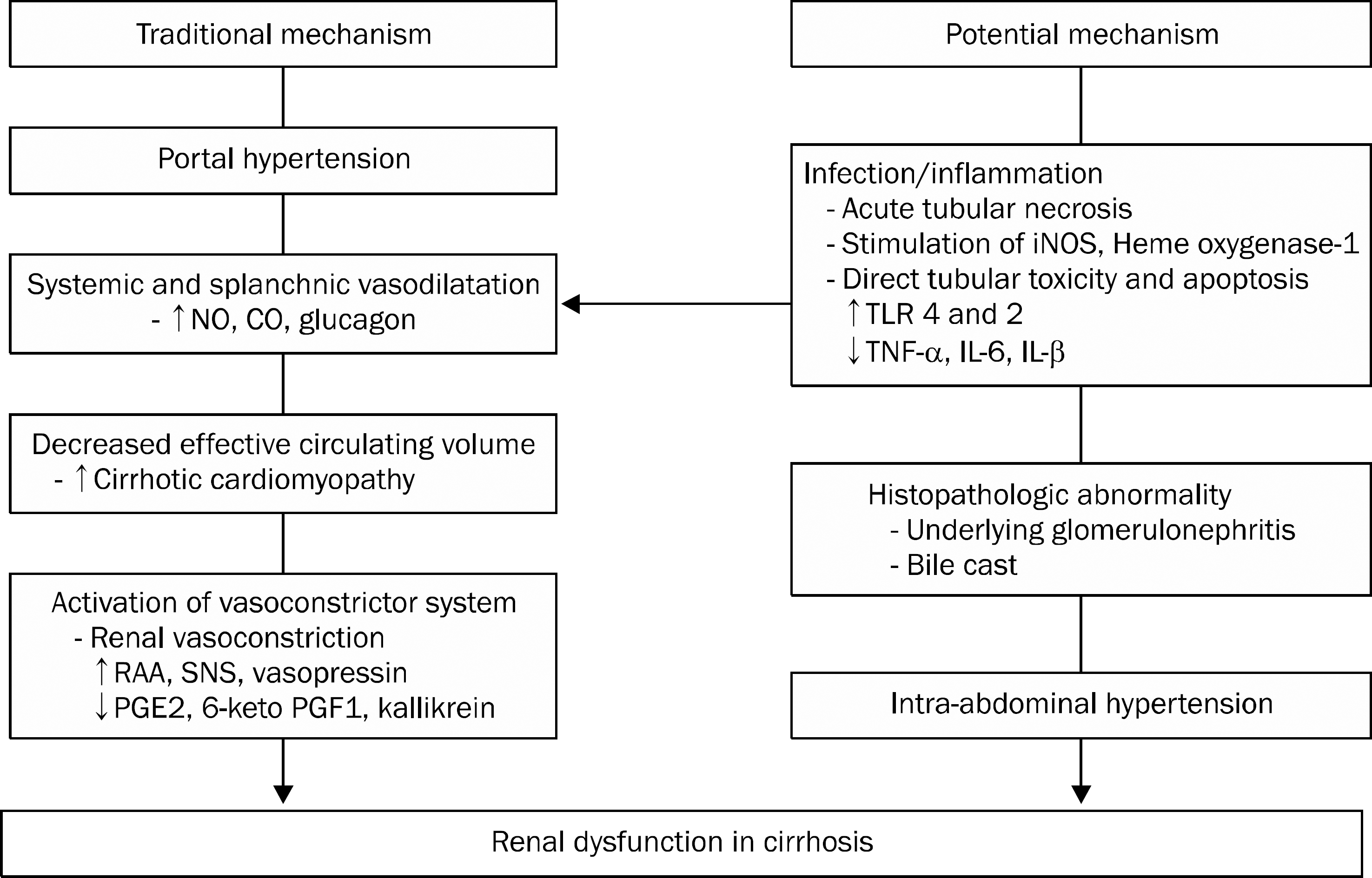 | Fig. 1.Traditional and potential mechanisms of the development of acute kidney injury in cirrhotic patients with portal hypertension. NO, nitric oxide; CO, carbon monoxide; iNOS, inducible NO; TLR, toll like receptor; TNF, tumor necrosis factor; IL, interleukin; RAA, renin-angiotensin- aldosterone; SNS, sympathetic nervous system; PGE2, prostaglandin E2;6-keto PGF1, 6-keto-prostaglandin F1. |
Table 1.
A Comparison of the Diagnostic Criteria of AKI in General Population and Cirrhotic Patients
AKI, acute kidney injury; RIFLE, Risk, Injury, Failure, Loss, End-stage kidney disease; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease Improving Global Outcome; ICA, International Club of Ascites; sCr, serum creatinine; GFR, glomerular filtration rate; UO, urine output; RRT, renal replacement therapy.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download