Abstract
Epstein-Barr virus (EBV) infection varies in its clinical manifestations and severity. EBV can be a causative agent of hepatitis and may have a role in the pathogenesis of chronic autoimmune diseases including inflammatory bowel disease. A 24-year-old woman was admitted to our hospital, presenting with fever and elevated liver enzymes. She was diagnosed with acute hepatitis and EBV infection according to serologic tests and liver biopsy. Within two months, she was re-admitted to our hospital, presenting with hematochezia and lower abdominal pain. She was diagnosed with ulcerative colitis. In situ hybridization for EBV was positive in initial liver biopsy and colon biopsy. Here we report an unusual case of acute EBV hepatitis followed at a short interval by ulcerative colitis.
Go to : 
References
1. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lym-phoblasts from Burkitt's lymphoma. Lancet. 1964; 1:702–703.

2. James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997; 100:3019–3026.

3. Whittingham S, McNeilage LJ, Mackay IR. Epstein-Barr virus as an etiological agent in primary Sjögren's syndrome. Med Hypotheses. 1987; 22:373–386.

4. Gehlert T, Devergne O, Niedobitek G. Epstein-Barr virus (EBV) infection and expression of the interleukin-12 family member EBV-induced gene 3 (EBI3) in chronic inflammatory bowel disease. J Med Virol. 2004; 73:432–438.

5. Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999; 94:1582–1586.

6. Linton MS, Kroeker K, Fedorak D, Dieleman L, Fedorak RN. Prevalence of Epstein-Barr virus in a population of patients with inflammatory bowel disease: a prospective cohort study. Aliment Pharmacol Ther. 2013; 38:1248–1254.

8. Gupta E, Bhatia V, Choudhary A, Rastogi A, Gupta NL. Epstein-Barr virus associated acute hepatitis with cross-reacting antibodies to other herpes viruses in immunocompetent patients: report of two cases. J Med Virol. 2013; 8:519–523.

9. Donhuijsen-Ant R, Abken H, Westerhausen M, et al. Aggressive hepatitis in a patient with acute myeloid leukaemia during complete remission and detection of Epstein-Barr virus DNA in a liver biopsy. Br J Haematol. 1990; 76:557–558.

10. Odumade OA, Hogquist KA, Balfour HH Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011; 24:193–209.

11. Horwitz CA, Henle W, Henle G, et al. Clinical and laboratory evaluation of infants and children with Epstein-Barr virus-induced infectious mononucleosis: report of 32 patients (aged 10–48 months). Blood. 1981; 57:933–938.
12. Horwitz CA, Henle W, Henle G, Penn G, Hoffman N, Ward PC. Persistent falsely positive rapid tests for infectious mononucleosis. Report of five cases with four–six-year follow-up data. Am J Clin Pathol. 1979; 72:807–811.

13. Hess RD. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol. 2004; 42:3381–3387.

14. Thompson SK, Doerr TD, Hengerer AS. Infectious mononucleosis and corticosteroids: management practices and outcomes. Arch Otolaryngol Head Neck Surg. 2005; 131:900–904.
15. Candy B, Hotopf M. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst Rev. 2006; (3):CD004402.

16. Wakefield AJ, Fox JD, Sawyerr AM, et al. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn's disease using the nested polymerase chain reaction. J Med Virol. 1992; 38:183–190.

17. Ryan JL, Shen YJ, Morgan DR, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci. 2012; 57:1887–1898.

18. Rigopoulou EI, Smyk DS, Matthews CE, et al. Epstein-barr virus as a trigger of autoimmune liver diseases. Adv Virol. 2012; 2012:987471.

19. Pender MP. CD8+ T-Cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012; 2012:189096.

Go to : 
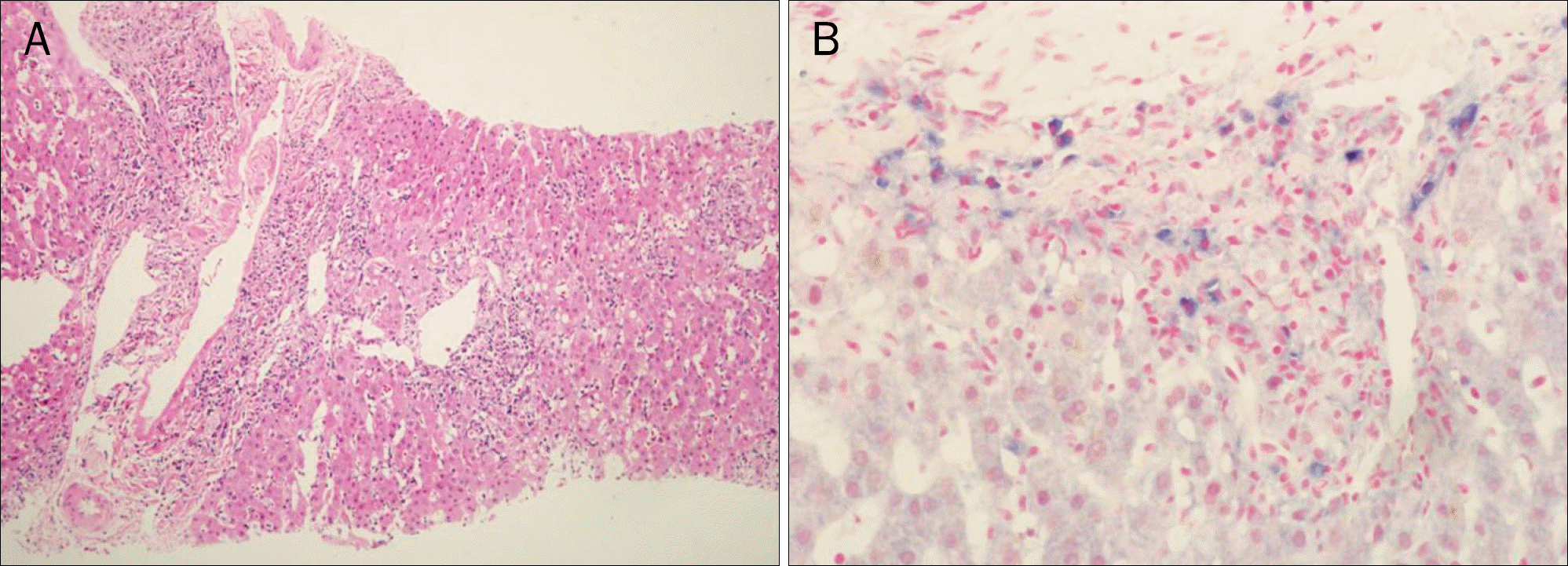 | Fig. 1.Initial liver biopsy findings. (A) H&E (×100). Intralobular focal necrosis and infiltration of inflammatory cells in portal area are shown. A linear sinusoidal lymphocytic infiltration is also seen. (B) Epstein-Barr virus in situ hybridization (×400). Positive staining cells (blue color) were detected within lymphocytes infiltrating in periportal area. |
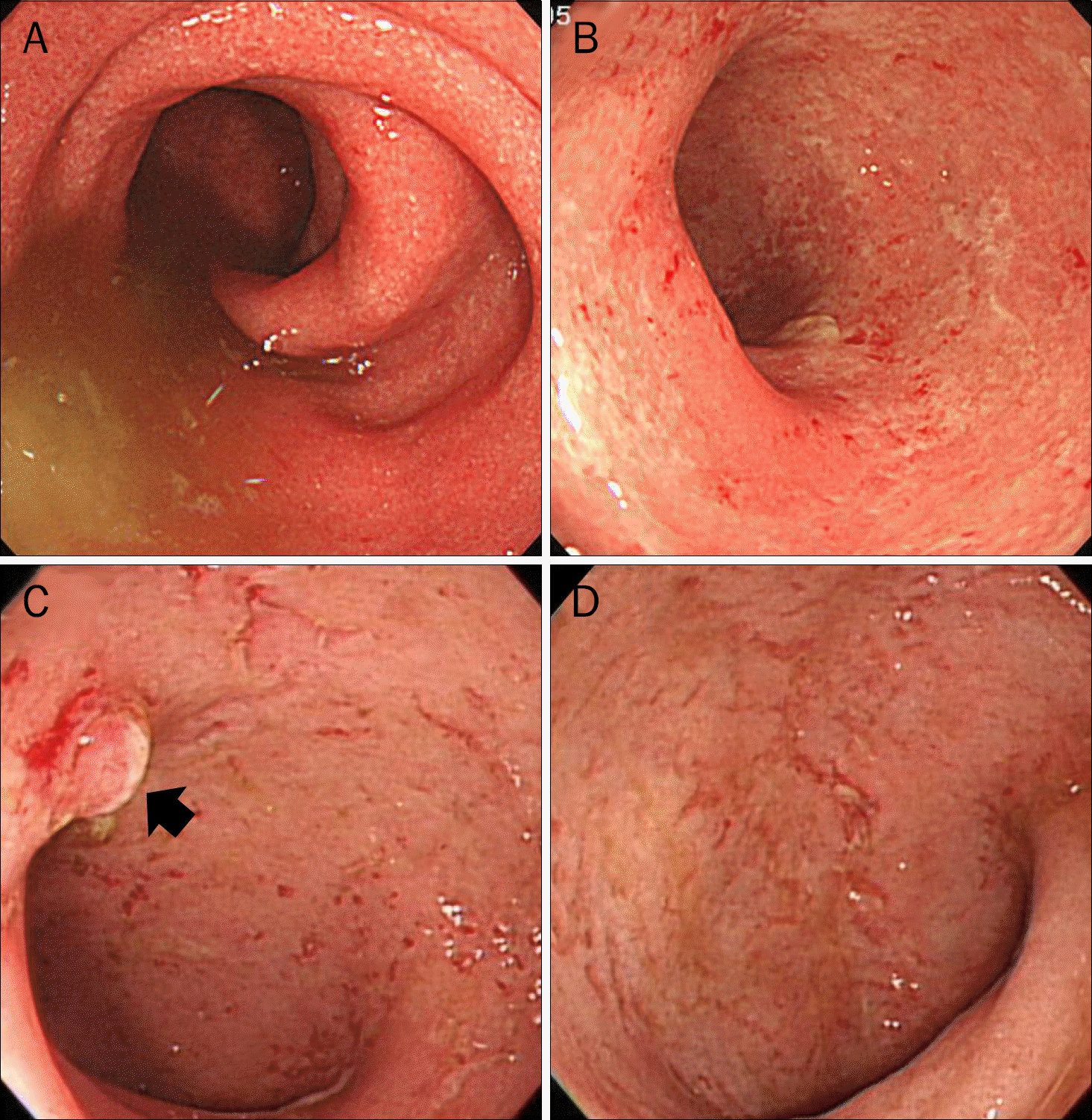 | Fig. 2.(A, B) Colonoscopic images of sigmoid colon at second admission. Loss of vascular pattern and some friability of the mucosa is seen. (C, D) Follow-up colonoscopic images of sigmoid colon and rectum after one year. This image shows multiple mucosal erosions, loss of vascular appearance and an inflammatory polyp (arrow). |
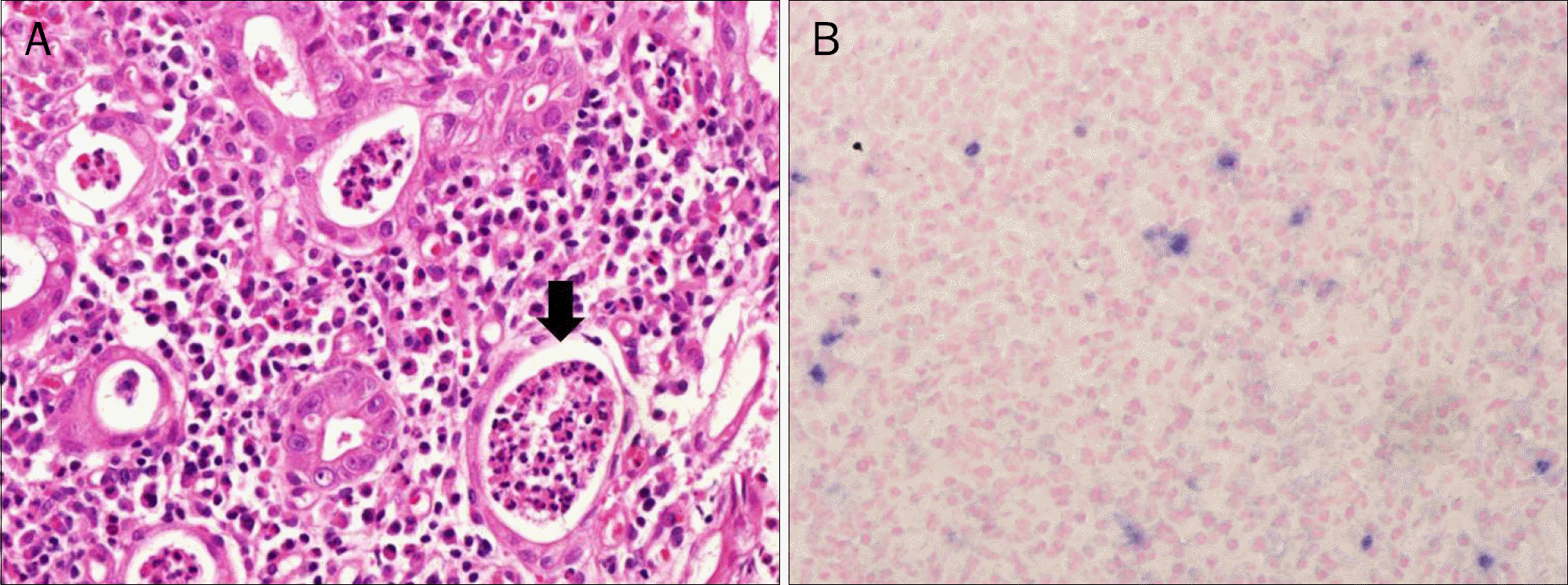 | Fig. 3.Microsopic findings of colon biopsy. (A) H&E (×400). The infiltration of mixed inflammatory cells and acute cryptitis (arrow) are shown. (B) Epstein-Barr virus in situ hybridization (×400). Positive staining cells (blue color) were detected within lymphocytes infiltrating in area of active inflammation. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download