Abstract
Background/Aims
Although intravenous proton pump inhibitor (PPI) has been used for the prevention of post endoscopic submucosal dissection (ESD) bleeding, the route of administration has not been confirmed. The aim of the present study was to compare the efficacy of intravenous and oral PPI administration for the prevention of delayed post ESD bleeding.
Methods
Total 166 consecutive patients were randomly assigned to 30 mg lansoprazol twice a day (PO group) and 120 mg pantoprazole intravenous injection (IV group) for 48 hours. Finally, 65 patients in PO group and 87 patients in IV group were analyzed. After ESD, all patients underwent follow up endoscopy after 24 hours and were observed the symptoms of bleeding up to 60 days after ESD.
Results
Age, sex and use of anticoagulants were not different between groups. At follow up endoscopy after 24 hours, oozing and exposed vessel was noted in 4.6% of PO group and 8.0% of IV group and there was no significant difference. Delayed bleeding occurred in 4 of 65 patients (6.2%) in the PO group and 8 of 87 patients (9.2%) in the IV group (p>0.999). By multivariate analysis, oozing or exposed vessels at follow up endoscopy were risk factors for delayed bleeding (OR=17.5, p=0.022).
Conclusions
There was no significant difference in the delayed bleeding, length of hospital stay according to the administration route. Bleeding stigmata at follow up endoscopy was risk factor of delayed bleeding. Oral PPI administration can cost-effectively replace IV PPI for prevention of post ESD bleeding.
Go to : 
References
1. Fujishiro M, Yahagi N, Kakushima N, et al. Successful non-surgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006; 38:1001–1006.

2. Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002; 55:390–396.

3. Ye BD, Cheon JH, Choi KD, et al. Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharmacol Ther. 2006; 24:837–843.

4. Yamaguchi Y, Katsumi N, Tauchi M, et al. A prospective randomized trial of either famotidine or omeprazole for the prevention of bleeding after endoscopic mucosal resection and the healing of endoscopic mucosal resectioninduced ulceration. Aliment Pharmacol Ther. 2005; 21(Suppl 2):111–115.

5. Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007; 102:1610–1616.

6. Jeong HK, Park CH, Jun CH, et al. A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J Korean Med Sci. 2007; 22:1055–1059.

7. Javid G, Zargar SA, U-Saif R, et al. Comparison of p.o. or i.v. proton pump inhibitors on 72-h intragastric pH in bleeding peptic ulcer. J Gastroenterol Hepatol. 2009; 24:1236–1243.

8. Jang JS, Choi SR, Graham DY, et al. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol. 2009; 44:1370–1376.

9. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009; 69:1228–1235.

10. Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008; 134:1836–1841.

11. Tsai JJ, Hsu YC, Perng CL, Lin HJ. Oral or intravenous proton pump inhibitor in patients with peptic ulcer bleeding after successful endoscopic epinephrine injection. Br J Clin Pharmacol. 2009; 67:326–332.

12. Murthy S, Keyvani L, Leeson S, Targownik LE. Intravenous versus highdose oral proton pump inhibitor therapy after endoscopic hemostasis of high-risk lesions in patients with acute non-variceal upper gastrointestinal bleeding. Dig Dis Sci. 2007; 52:1685–1690.

13. Mostaghni AA, Hashemi SA, Heydari ST. Comparison of oral and intravenous proton pump inhibitor on patients with high risk bleeding peptic ulcers: a prospective, randomized, controlled clinical trial. Iran Red Crescent Med J. 2011; 13:458–463.
14. Tsoi KK, Hirai HW, Sung JJ. Meta-analysis: comparison of oral vs. intravenous proton pump inhibitors in patients with peptic ulcer bleeding. Aliment Pharmacol Ther. 2013; 38:721–728.

15. Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003; 57:687–690.

16. Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection: an analysis of risk factors. Endoscopy. 2008; 40:179–183.
17. Tsuji Y, Ohata K, Ito T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010; 16:2913–2917.

18. Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012; 27:907–912.

19. Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012; 2012:875323.

20. Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013; 25(Suppl 1):71–78.

Go to : 
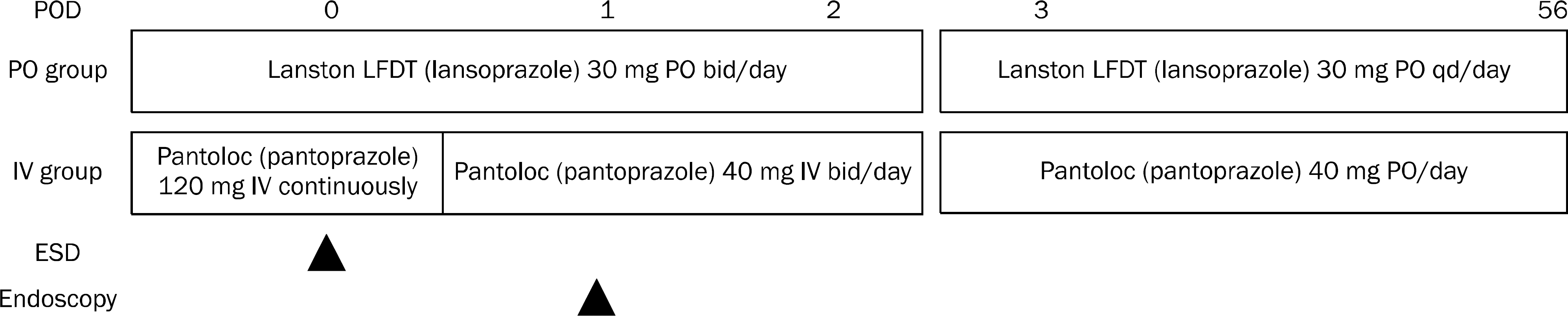 | Fig. 1.Treatment protocol of the groups. POD, post-operative day; PO, per oral; IV, intravenous; ESD, endoscopic submucosal dissection; bid, twice a day; qd, once a day. |
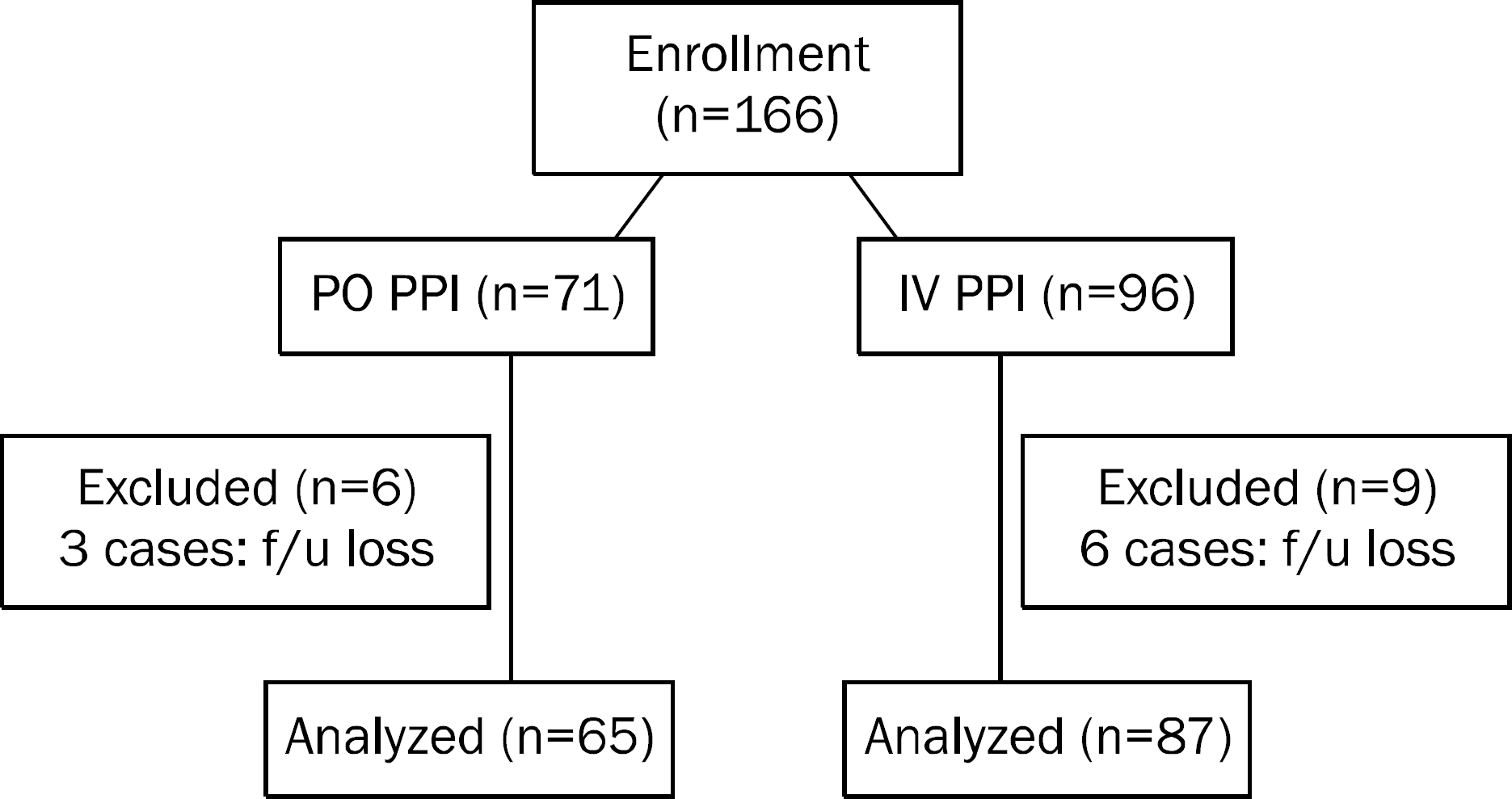 | Fig. 2.Flow chart of study participants. PO, per oral; IV, intravenous; PPI, proton pump inhibitor; f/u, follow up. |
Table 1.
Baseline Characteristics of 151 Analyzed Patients
| PO group (n=65) | IV group (n=87) | p-value | |
|---|---|---|---|
| Age (yr) | 62.0±8.8 | 62.6±9.6 | 0.702 |
| Male | 45 (69.2) | 64 (73.6) | 0.557 |
| Laboratory findings | |||
| Hemoglobin (g/dL) | 13.2±1.5 | 13.5±1.5 | 0.181 |
| BUN (mg/dL) | 15.1±5.2 | 14.9±6.0 | 0.884 |
| Creatinine (mg/dL) | 1.5±5.2 | 0.9±0.7 | 0.272 |
| Underlying disease | 36 (55.4) | 36 (41.4) | 0.087 |
| Diabetes mellitus | 10 (15.4) | 8 (9.2) | 0.243 |
| Hypertension | 24 (36.9) | 26 (29.9) | 0.361 |
| Cerebro-cardiovascular | 8 (12.3) | 2 (2.3) | 0.019 |
| Others* | 6 (9.2) | 7 (8.0) | 0.796 |
| Anticoagulation | 15 (23.1) | 13 (14.9) | 0.201 |
| Warfarin | 0 (0) | 0 (0) | − |
| Aspirin | 14 (21.5) | 13 (14.9) | 0.293 |
| Clopidogrel | 2 (3.1) | 2 (2.3) | >0.999 |
| Cilostazole | 1 (1.5) | 0 | 0.428 |
| Discontinuation period (day) | 9.1±6.2 | 7.5±1.7 | 0.352 |
Table 2.
Endoscopic and Microscopic Findings of the Lesion
Table 3.
Comparison of Bleeding after Endoscopic Submucosal Dissection
Table 4.
Comparisons between Rebleeding Group and Nonbleeding Group
Table 5.
Risk Factors Associated with Post ESD Symptomatic Bleeding (n=5)
Table 6.
Risk Factors Associated with Post ESD Symptomatic Bleeding after 24 Hours (n=3)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download