Abstract
Pegylated interferon alpha (PEG-IFN-α) is widely used to treat chronic hepatitis C in combination with ribavirin. Many adverse effects of PEG-IFN-α, such as hematologic, psychologic, dermatologic, immunologic, and other abnormalities, have been reported, and some serious adverse events lead to PEG-IFN-α treatment discontinuation. For very rare adverse events such as panniculitis, there are no established guidelines on whether to continue PEG-IFN-α treatment. Published reports on panniculitis induced by PEG-IFN-α 2a are sparse. Herein we report a case of repeated occurrences of panniculitis in a patient with chronic hepatitis C, leading to treatment cessation.
Go to : 
References
1. Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986; 315:1575–1578.
2. Sulkowski MS, Cooper C, Hunyady B, et al. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011; 8:212–223.

3. O'Sullivan SS, Cronin EM, Sweeney BJ, Bourke JF, Fitzgibbon J. Panniculitis and lipoatrophy after subcutaneous injection of interferon beta-1b in a patient with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006; 77:1382–1383.
4. Heinzerling L, Dummer R, Burg G, Schmid-Grendelmeier P. Panniculitis after subcutaneous injection of interferon beta in a multiple sclerosis patient. Eur J Dermatol. 2002; 12:194–197.
5. Poulin F, Rico P, Côté J, Bégin LR. Interferon beta-induced panniculitis mimicking acute appendicitis. Arch Dermatol. 2009; 145:916–917.

6. Baek YS, Kim JY, Choi JE, Ahn HH, Kye YC, Seo SH. Pegylated interferon Alpha2a induced panniculitis in patient with chronic hepatitis C. Korean J Dermatol. 2012; 50:640–643.
7. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998; 352:1426–1432.
8. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998; 339:1485–1492.
9. Glue P, Fang JW, Rouzier-Panis R, et al. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin Pharmacol Ther. 2000; 68:556–567.
10. Bailon P, Palleroni A, Schaffer CA, et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001; 12:195–202.
11. Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997; 26(3 Suppl 1):112S–121S.
14. Buttmann M, Goebeler M, Toksoy A, et al. Subcutaneous interferon-beta injections in patients with multiple sclerosis initiate inflammatory skin reactions by local chemokine induction. J Neuroimmunol. 2005; 168:175–182.
15. Inafuku H, Kasem Khan MA, Nagata T, Nonaka S. Cutaneous ulcerations following subcutaneous interferon beta injection to a patient with multiple sclerosis. J Dermatol. 2004; 31:671–677.
Go to : 
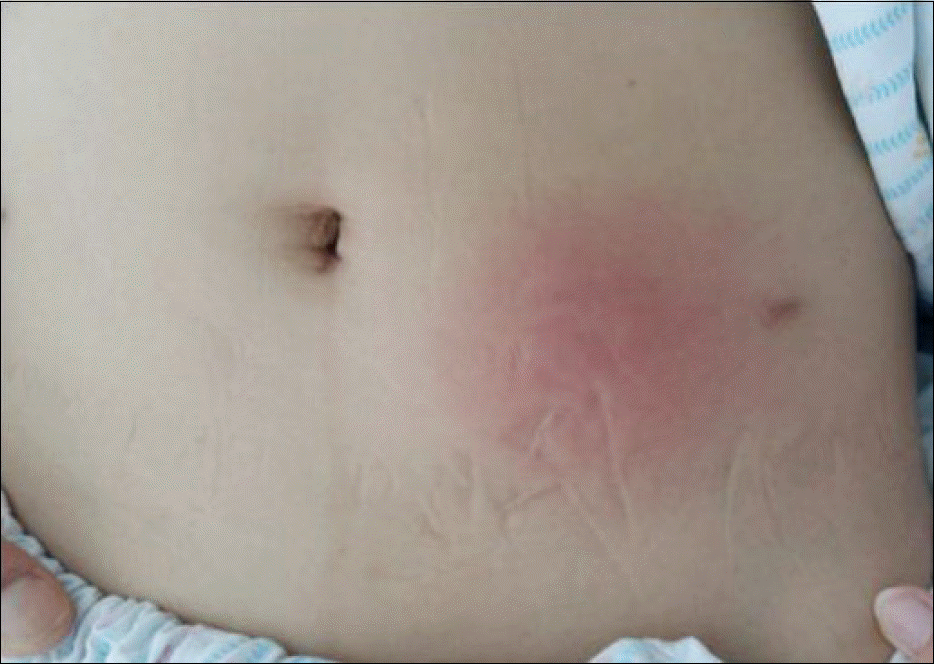 | Fig. 1.The skin lesion was reddish and edematous, and the size of subcutaneous nodule was 6×6 cm. |
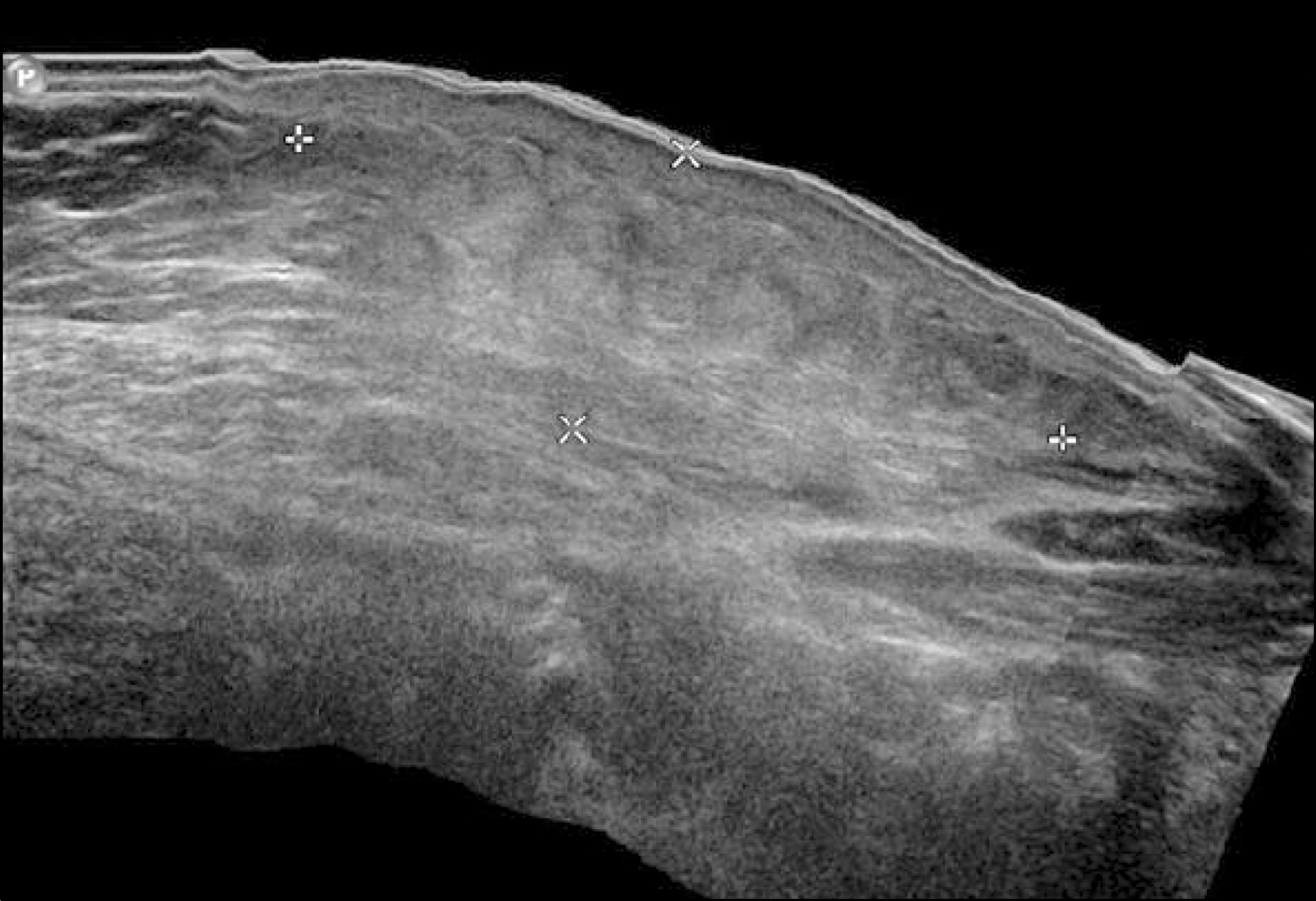 | Fig. 2.Ultrasonographic findings. Soft tissue ultrasound shows cellulitis, panniculitis and myositis of the left abdominal wall compatible with chemical-induced inflammation. |
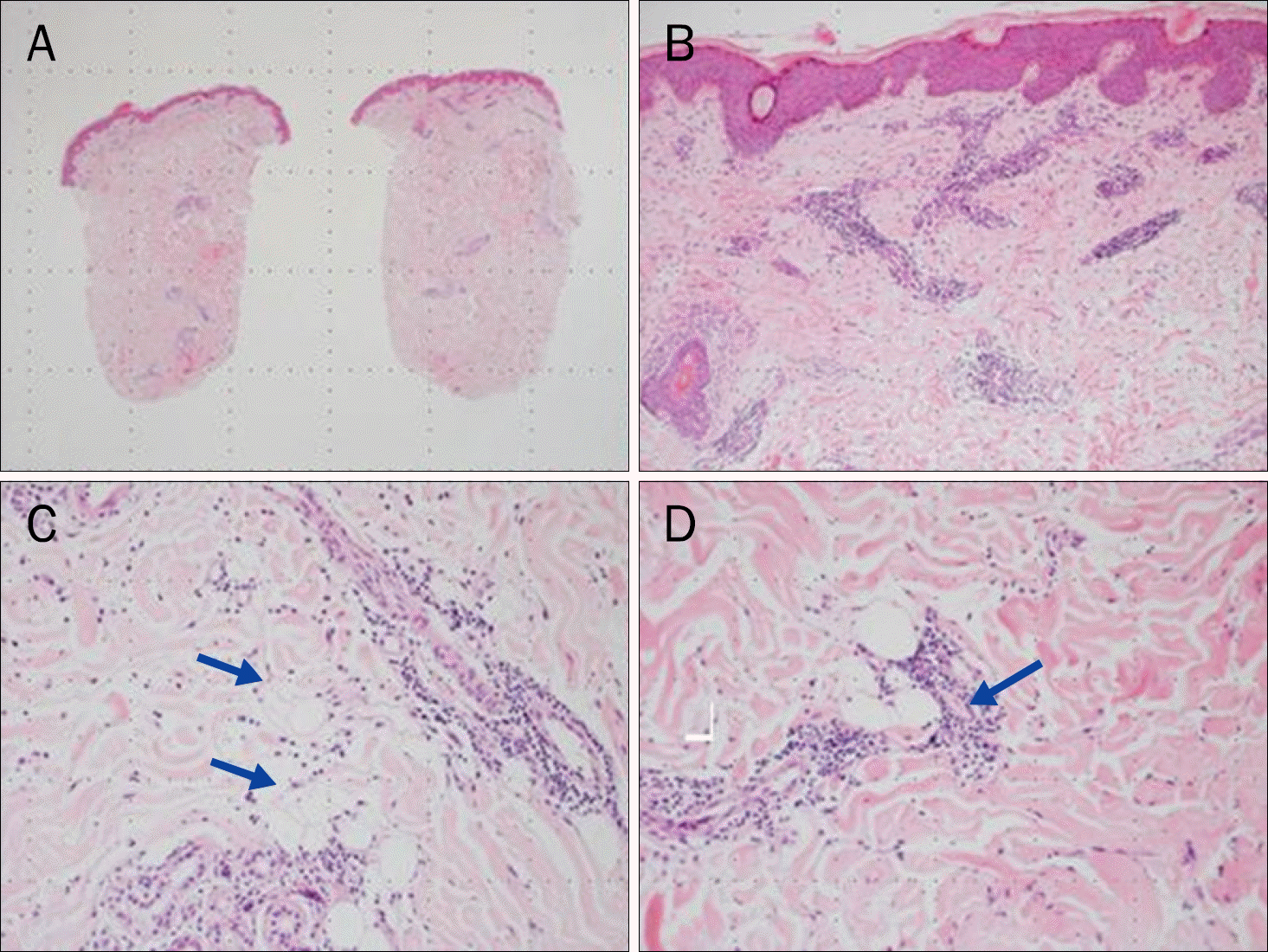 | Fig. 3.Microscopic findings of the punch biopsy specimen (H&E). (A) Histology of bisected tissue section of abdominal skin lesion (×12.5). (B) Superficial perivascular and interstitial lymphocytic infiltrates (×100). (C) Deep perivascular and interstitial lymphocytic infiltrates (×200) (arrows).(D) In deep dermis, a few degenerated adipocytes with lymphocytic infiltration (×200) (arrow). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download