Abstract
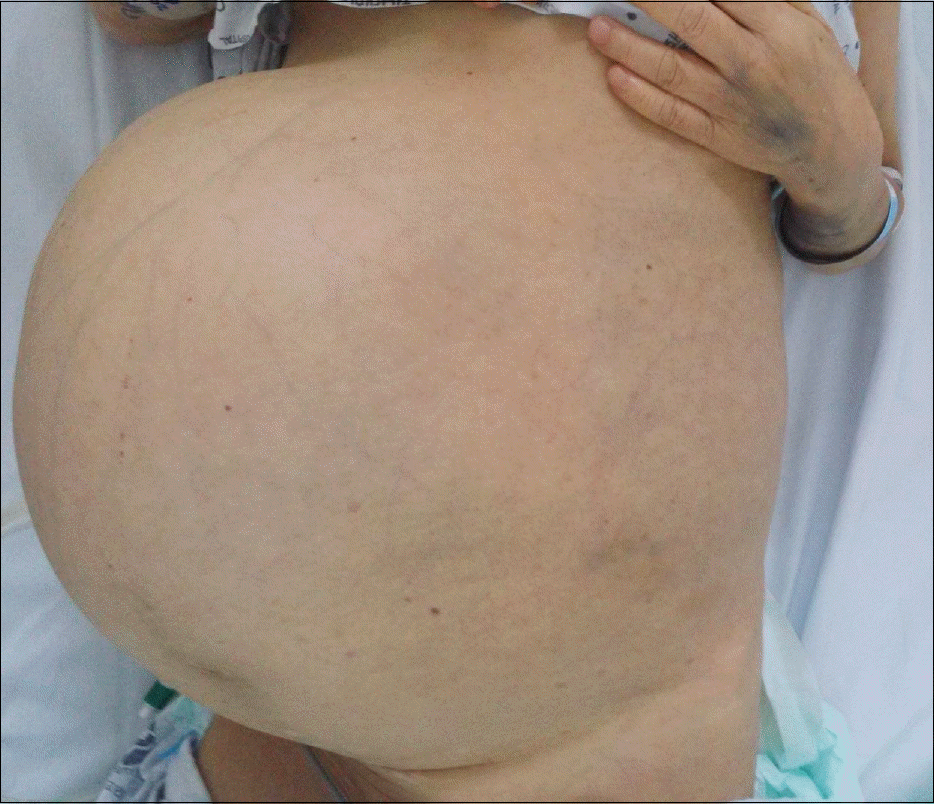
Hemangiomas are the most common benign tumors of the liver. They are generally asymptomatic, but giant hemangiomas can lead to abdominal discomfort, bleeding, or obstructive symptoms. KasabachMerritt syndrome is a rare but life-threatening complication of hemangioma, characterized by consumptive coagulopathy with large vascular tumors. More than 80% of KasabachMerritt syndrome cases occur within the first year of life. However, there are few reports of KasabachMerritt syndrome with giant hepatic hemangioma in adults and, as far as we know, no reports of KasabachMerritt syndrome with hepatic hemangioma treated with first line medical treatment only. The most important treatment for this syndrome is removal of the large vascular tumor. However, surgical treatment entails risk of bleeding, and the patient's condition can mitigate against surgery. We herein present a case of unresectable giant hepatic hemangioma with disseminated intravascular coagulopathy. The patient was a 60-year-old woman who complained of hematochezia, ecchymosis, and abdominal distension. She refused all surgical management and was therefore treated with systemic glucocorticoids and beta-blockers. After two weeks of steroid therapy, she responded partially to the treatment. Her laboratory findings and hematochezia improved. She was discharged on hospital day 33 and observed without signs of bleeding for three months.
References
2. Choi BY, Nguyen MH. The diagnosis and management of benign hepatic tumors. J Clin Gastroenterol. 2005; 39:401–412.

4. Hall GW. KasabachMerritt syndrome: pathogenesis and management. Br J Haematol. 2001; 112:851–862.

5. Kasabach HH, Merritt KK. Capillary hemangioma with extensive purpura. Am J Dis. 1940; 59:1063–1070.

6. Enjolras O, Wassef M, Mazoyer E, et al. Infants with KasabachMerritt syndrome do not have "true" hemangiomas. J Pediatr. 1997; 130:631–640.

7. Hochwald SN, Blumgart LH. Giant hepatic hemangioma with KasabachMerritt syndrome: is the appropriate treatment enucleation or liver transplantation? HPB Surg. 2000; 11:413–419.
8. Meguro M, Soejima Y, Taketomi A, et al. Living donor liver transplantation in a patient with giant hepatic hemangioma complicated by KasabachMerritt syndrome: report of a case. Surg Today. 2008; 38:463–468.

9. Malagari K, Alexopoulou E, Dourakis S, et al. Transarterial embolization of giant liver hemangiomas associated with KasabachMerritt syndrome: a case report. Acta Radiol. 2007; 48:608–612.

10. Aslan A, Meyer Zu Vilsendorf A, Kleine M, Bredt M, Bektas H. Adult Kasabach-merritt syndrome due to hepatic giant hemangioma. Case Rep Gastroenterol. 2009; 3:306–312.

11. McKay MJ, Carr PJ, Langlands AO. Treatment of hepatic cavernous haemangioma with radiation therapy: case report and literature review. Aust N Z J Surg. 1989; 59:965–968.

12. el-Dessouky M, Azmy AF, Raine PA, Young DG. KasabachMerritt syndrome. J Pediatr Surg. 1988; 23:109–111.

14. Seo SK, Suh JC, Na GY, Kim IS, Sohn KR. KasabachMerritt syndrome: identification of platelet trapping in a tufted angioma by immunohistochemistry technique using monoclonal antibody to CD61. Pediatr Dermatol. 1999; 16:392–394.
Fig. 2.
Abdominal CT scan at presentation with several centripetal enhancing exophytic masses in both hepatic lobes (maximum size, 32.3×27.5×25.4 cm). (A) Cross section image. (B) Coronal section image. (C) Cross section image of abdominal CT 8 years prior (maximal size, 22.3 cm).
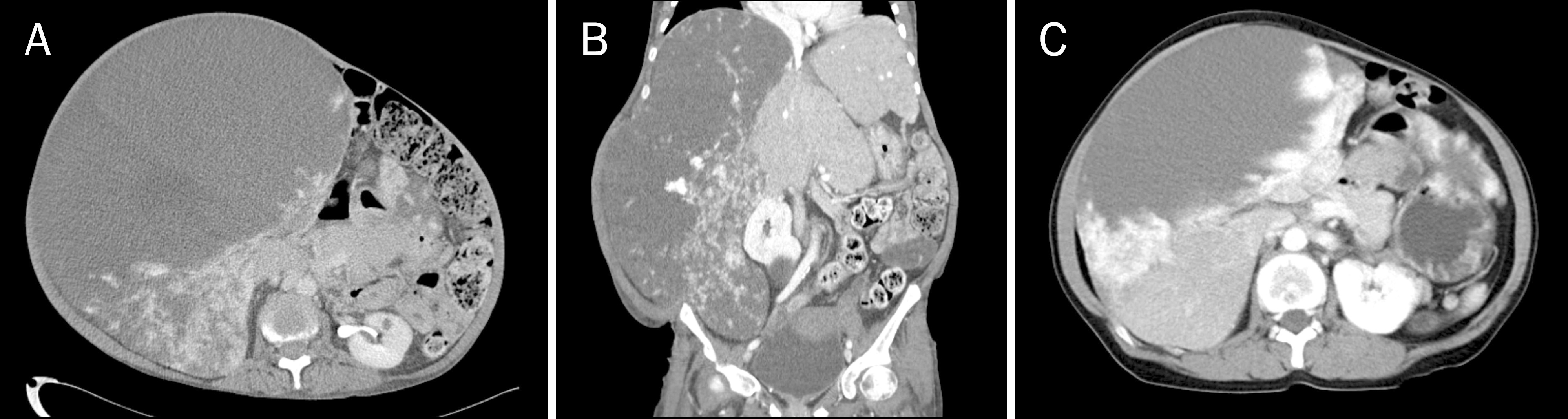
Table 1.
Complete Blood Count and Coagulation Profile during Hospital Stay
| HD 1 a | HD 3 a | HD 5 a (steroid+1) | HD 10 a (steroid+6) | HD 16(steroid+12) | HD 23(steroid+19) | HD 29(steroid+25) | |
|---|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 8.2 | 7.5 | 9.1 | 8.8 | 9.0 | 7.9 | 8.4 |
| Platelet (/mm3) | 32,000 | 29,000 | 36,000 | 39,000 | 40,000 | 54,000 | 92,000 |
| PT (sec) | 28.0 | 15.7 | 16.9 | >100 | 18.0 | 14.2 | 13.1 |
| aPTT (sec) | >100 | 51.6 | 41.8 | >100 | 39.8 | 37.4 | 40.0 |
| Fibrinogen (mg/dL) | <50 | 59.1 | 46.2 | <50 | <50 | 60 | 109.1 |
| FDP (μ g/mL) | 115.2 | 184.3 | 268.6 | 241.7 | 137.0 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download