Abstract
Background/Aims
Obscure gastrointestinal bleeding (OGIB) accounts for 5% of all gastrointestinal (GI) bleeding cases. Dynamic contrast-enhanced multidetector-row CT (DCE-MDCT) is not generally recommended in OGIB patients due to its low sensitivity. However, it can be used to quickly and simply diagnose OGIB according to some guidelines. The aim of this study was to evaluate the clinical efficacy of DCE-MDCT in OGIB patients.
Methods
We retrospectively analyzed the medical records of 362 patients who underwent DCE-MDCT between March 2009 and January 2014. A total of 45 patients diagnosed with OGIB were included in this study. Their baseline characteristics and treatment procedure were analyzed retrospectively. The positive rates of DCE-MDCT for the detection of bleeding and associated factors were assessed.
Results
The mean age of the patients was 59 years, and males represented 51.1%. Melena was the most common symptom (44.4%). Positive rate of DCE-MDCT findings was 20.0% (9/45). Among these patients, intraluminal contrast extravasation was found in 5 patients (55.6%) and intraluminal hematoma or mass lesions were found in 2 patients each (22.2%). Thirty nine patients (86.7%) underwent conservative management, and 6 patients (13.3%) underwent specific treatment, such as endoscopic treatment, embolization, or surgery. Patients who showed positivity in DCE-MDCT more frequently received specific treatment compared with those who were negative (44.4% vs. 5.6%, p=0.010).
References
1. García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J. EBM-Connect Collaboration. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and metaanalysis. Eur Radiol. 2013; 23:1181–1190.

2. Artigas JM, Martí M, Soto JA, Esteban H, Pinilla I, Guillén E. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. Radiographics. 2013; 33:1453–1470.

3. Rondonotti E, Marmo R, Petracchini M, de Franchis R, Pennazio M. The American Society for Gastrointestinal Endoscopy (ASGE) diagnostic algorithm for obscure gastrointestinal bleeding: eight burning questions from everyday clinical practice. Dig Liver Dis. 2013; 45:179–185.

4. Keum B, Chun HJ. Capsule endoscopy and double balloon enteroscopy for obscure gastrointestinal bleeding: which is better? J Gastroenterol Hepatol. 2011; 26:794–795.

5. Yoon W, Jeong YY, Shin SS, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multidetector row helical CT. Radiology. 2006; 239:160–167.

6. Sun H, Jin Z, Li X, et al. Detection and localization of active gastrointestinal bleeding with multidetector row computed tomography angiography: a 5-year prospective study in one medical center. J Clin Gastroenterol. 2012; 46:31–41.
7. Saperas E, Dot J, Videla S, et al. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007; 102:731–737.

8. Shim KN, Moon JS, Chang DK, et al. Korean Gut Image Study Group. Guideline for capsule endoscopy: obscure gastrointestinal bleeding. Clin Endosc. 2013; 46:45–53.

9. Fisher L, Lee Krinsky M, Anderson MA, et al. ASGE Standards of Practice Committee. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010; 72:471–479.

10. Graça BM, Freire PA, Brito JB, Ilharco JM, Carvalheiro VM, Caseiro-Alves F. Gastroenterologic and radiologic approach to obscure gastrointestinal bleeding: how, why, and when? Radiographics. 2010; 30:235–252.

11. Filippone A, Cianci R, Milano A, Pace E, Neri M, Cotroneo AR. Obscure and occult gastrointestinal bleeding: comparison of different imaging modalities. Abdom Imaging. 2012; 37:41–52.

12. Pasha SF, Hara AK, Leighton JA. Diagnostic evaluation and management of obscure gastrointestinal bleeding: a changing paradigm. Gastroenterol Hepatol (N Y). 2009; 5:839–850.
13. Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008; 68:1122–1127.
14. Saurin JC, Delvaux M, Gaudin JL, et al. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003; 35:576–584.

15. Pennazio M, Eisen G, Goldfarb N. ICCE. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy. 2005; 37:1046–1050.

16. Huprich JE, Fletcher JG, Alexander JA, Fidler JL, Burton SS, McCullough CH. Obscure gastrointestinal bleeding: evaluation with 64-section multiphase CT enterography–initial experience. Radiology. 2008; 246:562–571.

17. Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y. CT enterography in obscure gastrointestinal bleeding: a systematic review and metaanalysis. J Med Imaging Radiat Oncol. 2013; 57:263–273.

18. Kulkarni C, Moorthy S, Sreekumar K, et al. In the workup of patients with obscure gastrointestinal bleed, does 64-slice MDCT have a role? Indian J Radiol Imaging. 2012; 22:47–53.

19. Triester SL, Leighton JA, Leontiadis GI, et al. A metaanalysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005; 100:2407–2418.

20. Zhang BL, Jiang LL, Chen CX, Zhong BS, Li YM. Diagnosis of obscure gastrointestinal hemorrhage with capsule endoscopy in combination with multiple-detector computed tomography. J Gastroenterol Hepatol. 2010; 25:75–79.

21. Singh A, Baptista V, Stoicov C, Cave DR. Evaluation of small bowel bleeding. Curr Opin Gastroenterol. 2013; 29:119–124.

22. Kitiyakara T, Selby W. Non-small-bowel lesions detected by capsule endoscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005; 62:234–238.

23. Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Investigators. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005; 3:133–141.

Fig. 1.
Patient enrollment. Data on a total of 45 patients were collected retrospectively. DCE-MDCT, dynamic contrast-enhanced multidetector-row CT; GI, gastrointestinal; EGD, esophagogastroduodenoscopy; CFS, colonoscopy.
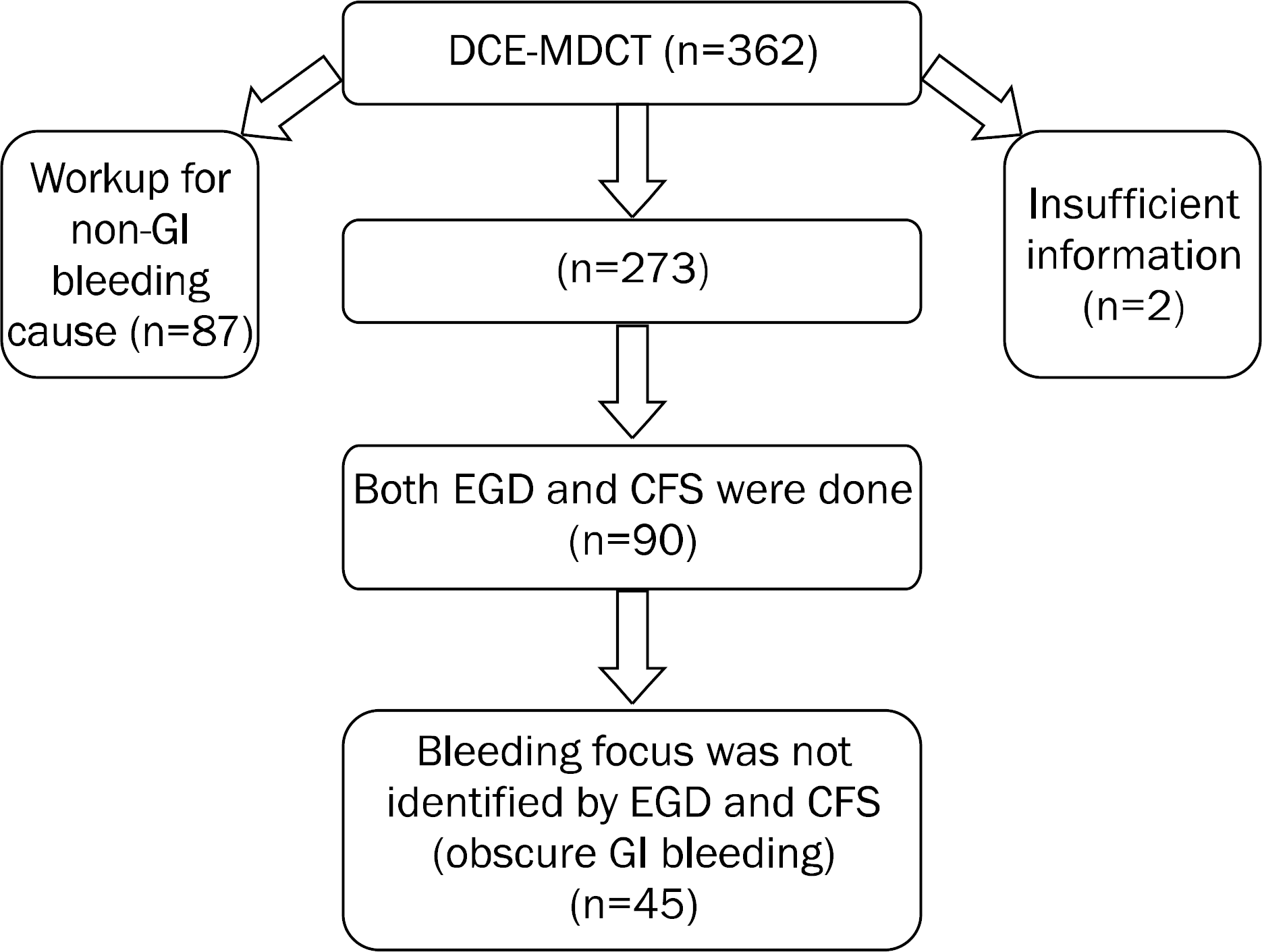
Fig. 2.
Dynamic contrast-enhanced multidetector-row CT image of a 61-year-old female with recurrent hematochezia. (A) Intraluminal contrast extravastion was noted in ileal loop (arrow). (B) Subsequent angiography revealed active contrast extravasation from ileal artery distal branch (arrow). (C) Successful arterial embolization was done (arrow).

Fig. 3.
Dynamic contrast-enhanced multidetector-row CT and capsule endoscopic image of a 73-year-old male with hematochezia. (A) Hyper-attenuating blood clot was visualized in ascending colon (asterisk). (B) Active ulcer (arrow) with fresh and old blood clot (arrowhead) was seen on the middle jejunum. Conservative management was performed and he did not experience recurrent bleeding after discontinuation of aspirin.
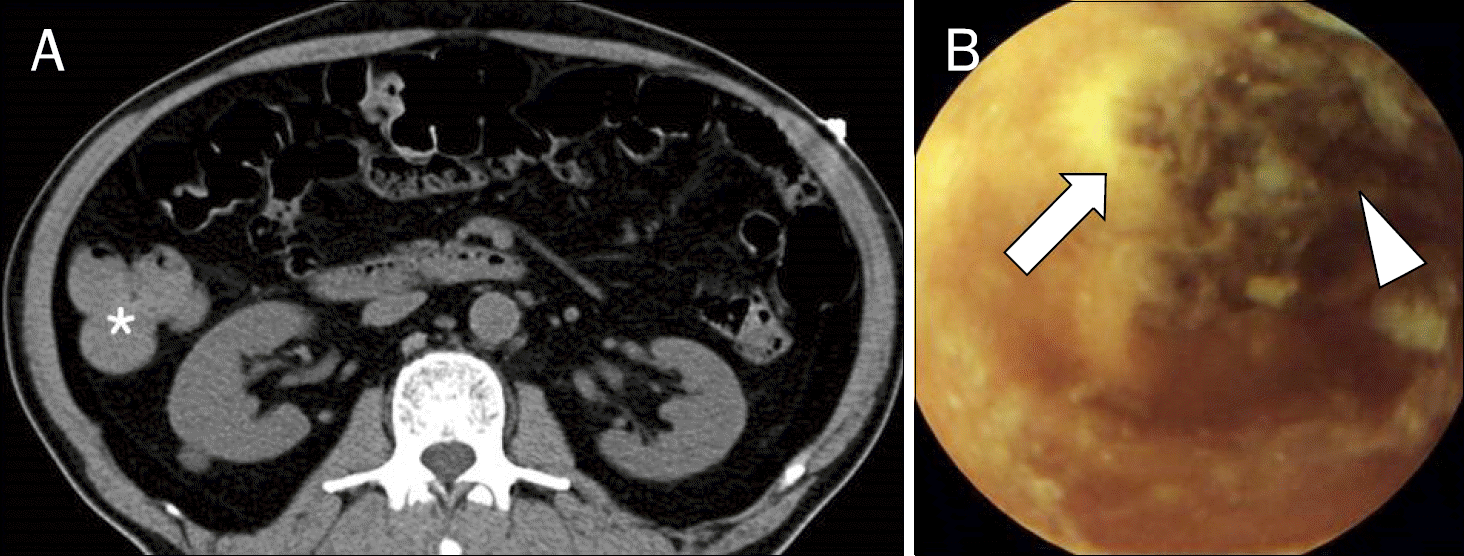
Fig. 4.
Dynamic contrast-enhanced multidetector-row CT (DCE-MDCT) and capsule endoscopic image of a 17-year-old male with recurrent iron deficiency anemia. (A) A small bowel hemangioma (arrow) with heterogeneous enhancement and small phleboliths were noted in the left side of pelvic cavity on DCE-MDCT. (B) A large pedunculated mass (arrows) with purplish surface was noted in the middle jejunum on capsule endoscopy. Surgical resection of the small bowel hemangioma was performed.

Fig. 5.
(A) Cumulative rebleeding rates of patients. (B) Cumulative rebleeding rates according to dynamic contrast-enhanced multidetector-row CT results.
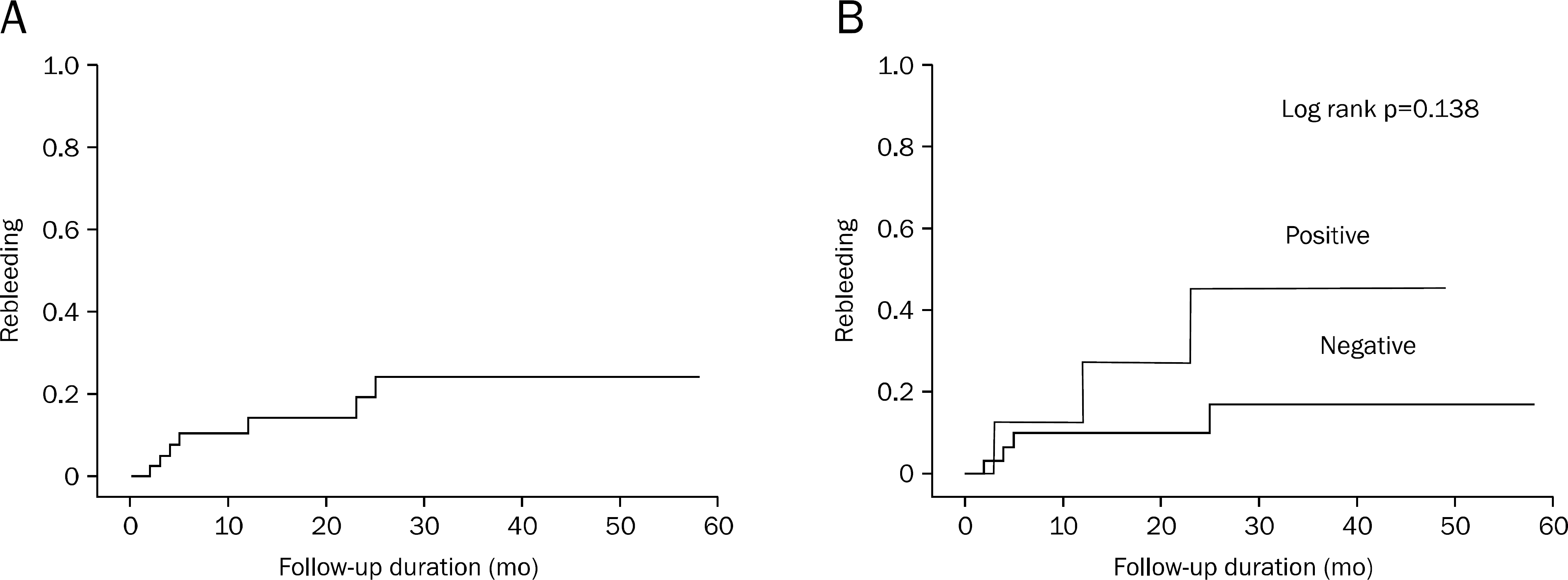
Table 1.
Baseline Characteristics of the Patients (n=45)
Table 2.
Clinical Characteristics of DCE-MDCT Positive Nine Patients
Table 3.
Comparison of Clinical Characteristics according to DCE-MDCT Results
Table 4.
Clinical Characteristics of Seven Patients with Recurrent Bleeding




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download