Abstract
Background/Aims
There are no studies that looked into the bubble eliminating efficacy of polyethylene glycol with ascorbic acid (PEGA), which has been one of the shortcomings of polyethylene glycol (PEG). In this study, we compared newly introduced PEGA regimen by adding either simethicone or 1 L of water.
Methods
A prospective randomized controlled study was carried out at Dongguk Universtiy Gyeongju Hospital from July 2014 to September 2014. A total of 90 patients were randomly assigned to 3 groups; PEGA group (n=30) which served as control, simethicone addition group (n=30) to which simethicone 400 mg was additionally prescribed, and water addition group (n=30) to whom additional 1 L of water was given. Cleansing effectiveness, gas elimination efficacy, side effects, and patient satisfaction were compared between the groups.
Results
PEGA group demonstrated the highest cleansing effectiveness, but there was no statistically significant difference among the groups. Simethicone addition group showed significantly lesser amount of bubbles than the other groups (2.57±2.05 vs. 1.10±1.83 vs. 2.60±2.84, p=0.017). The rates of side effects in each group were 20.00% vs. 16.77% vs. 53.33%. Water addition group had significantly more side effects than the PEGA group and the simethicone addition group (p=0.003). The patient satisfaction score of each group was 3.37±0.85 vs. 3.73±0.74 vs. 3.20±0.66 with simethicone addition group showing significantly higher satisfaction than water addition group (p=0.020).
Go to : 
References
1. Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA. 2003; 289:1288–1296.
2. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000; 343:162–168.

3. Leufkens AM, van Oijen MG, Vleggaar FP, Siersema PD. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy. 2012; 44:470–475.

4. Choi NK, Lee J, Chang Y, et al. Polyethylene glycol bowel preparation does not eliminate the risk of acute renal failure: a population-based case-crossover study. Endoscopy. 2013; 45:208–213.

5. Yoon JH, Park DI, Shin JE, et al. Comparison of bowel preparation depending on completion time of polyethylene glycol ingestion and start time of colonoscopy. Intest Res. 2010; 8:24–29.

6. DiPalma JA, Brady CE 3rd. Colon cleansing for diagnostic and surgical procedures: polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol. 1989; 84:1008–1016.
7. Tongprasert S, Sobhonslidsuk A, Rattanasiri S. Improving quality of colonoscopy by adding simethicone to sodium phosphate bowel preparation. World J Gastroenterol. 2009; 15:3032–3037.

8. Harewood GC, Wiersema MJ, Melton LJ 3rd. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002; 97:3186–3194.

9. Park JB, Lee YK, Yang CH. The evolution of bowel preparation and new developments. Korean J Gastroenterol. 2014; 63:268–275.

10. Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-vol-ume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008; 103:883–893.

11. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. 2011; 23:897–902.
12. Pontone S, Angelini R, Standoli M, et al. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol. 2011; 17:4689–4695.
13. Marmo R, Rotondano G, Riccio G, et al. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc. 2010; 72:313–320.

14. Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. 2010; 45:1380–1386.

15. Parikh VA, Khanduja KS. Use of simethicone during colonoscopy. Dis Colon Rectum. 1995; 38:1007–1008.

16. Nahm DI, Kim JB, Jung SW, et al. The effect of simethicone as a bowel preparative: is a higher dosage more helpful? Korean J Gastrointest Endosc. 2007; 34:251–255.
17. Shaver WA, Storms P, Peterson WL. Improvement of oral colonic lavage with supplemental simethicone. Dig Dis Sci. 1988; 33:185–188.

18. Sudduth RH, DeAngelis S, Sherman KE, McNally PR. The effectiveness of simethicone in improving visibility during colonoscopy when given with a sodium phosphate solution: a double-bind randomized study. Gastrointest Endosc. 1995; 42:413–415.
19. Tjandra JJ, Chan M, Tagkalidis PP. Oral sodium phosphate (Fleet) is a superior colonoscopy preparation to Picopre (sodium pico-sulfate-based preparation). Dis Colon Rectum. 2006; 49:616620.
20. Huh JG, Kim YS, Park JH, et al. A prospective comparison of sulfate free polyethylene glycol versus sodium phosphate solution for precolonoscopic bowel preparation. Korean J Gastrointest Endosc. 2009; 39:265–270.
21. Wexner SD, Beck DE, Baron TH, et al. American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006; 63:894–909.

22. Lazzaroni M, Petrillo M, Desideri S, Bianchi Porro G. Efficacy and tolerability of polyethylene glycol-electrolyte lavage solution with and without simethicone in the preparation of patients with inflammatory bowel disease for colonoscopy. Aliment Pharmacol Ther. 1993; 7:655–659.

23. Saltzman JR, Cash BD, Pasha SF, et al. ASGE Standards of Practice Committee. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015; 81:781–794.

24. Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet PhosphoSoda. Gastrointest Endosc. 2000; 52:346–352.

25. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009; 69:620–625.

26. Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003; 58:76–79.

27. Hong SN, Sung IK, Kim JH, et al. The effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy: a tandem colonoscopic study. Clin Endosc. 2012; 45:404–411.

28. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011; 73:1207–1214.

29. Kim CJ, Jung YS, Park JH, et al. Prevalence, clinicopathologic characteristics, and predictors of interval colorectal cancers in Korean population. Intest Res. 2013; 11:178–183.

30. Cha JM. Colonoscopy quality is the answer for the emerging issue of interval cancer. Intest Res. 2014; 12:110–116.

31. Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc. 2007; 66:565–573.

32. Moon CM, Park DI, Choe YG, et al. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol. 2014; 29:1223–1228.
33. McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988; 34:255258.

34. Munroe CA, Lee P, Copland A, et al. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc. 2012; 75:561–567.

35. Rex DK, Bond JH, Winawer S, et al. U.S. MultiSociety Task Force on Colorectal Cancer. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. MultiSociety Task Force on Colorectal Cancer. Am J Gastroenterol. 2002; 97:1296–1308.
36. Choi HN, Kim HH, Oh JS, et al. Factors influencing the miss rate of polyps in a tandem colonoscopy study. Korean J Gastroenterol. 2014; 64:24–30.

37. Soweid AM, Kobeissy AA, Jamali FR, et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy. 2010; 42:633–638.

Go to : 
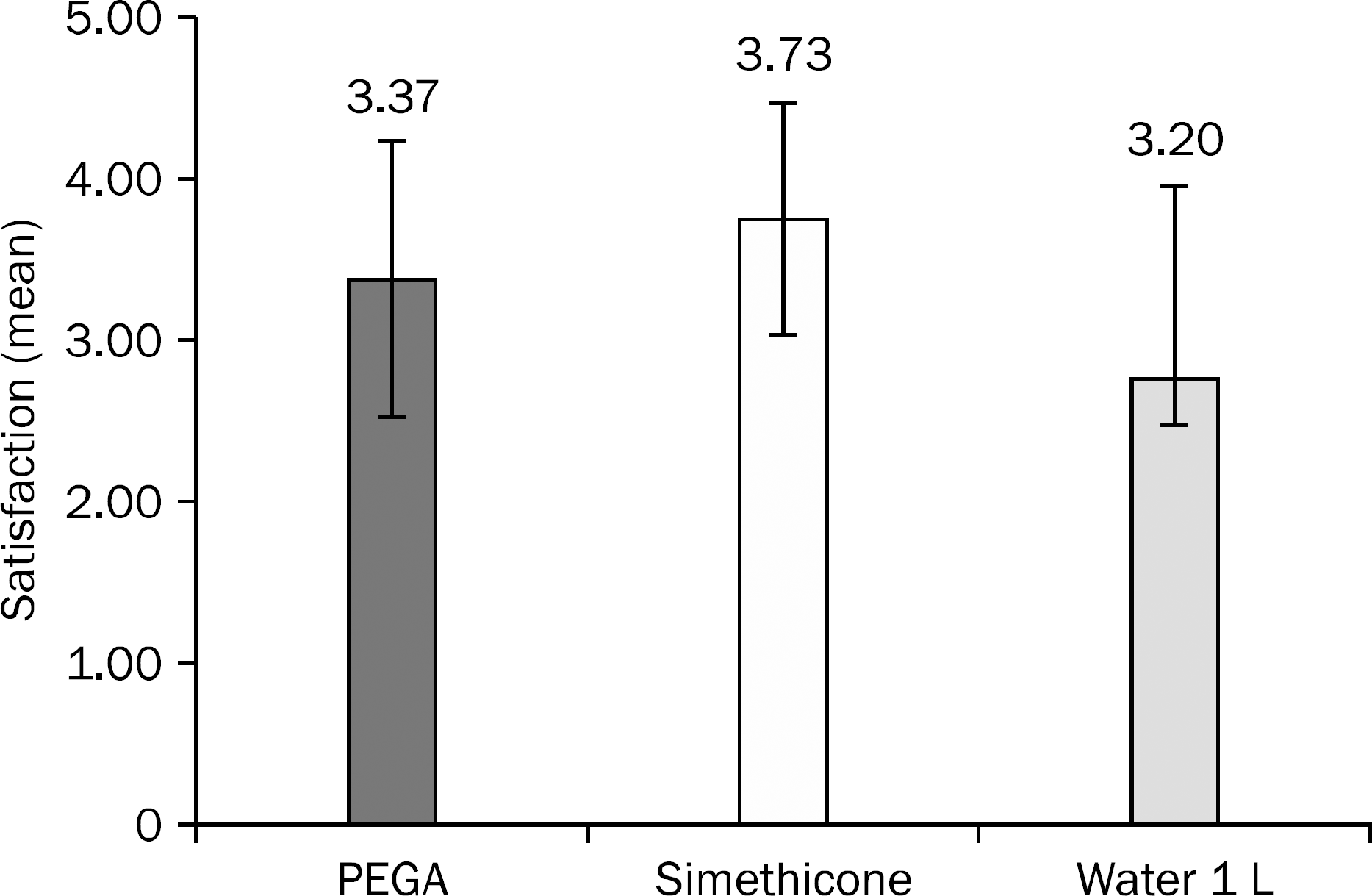 | Fig. 3.Satisfaction of bowel preparation methods. We investigated the satisfaction with a five-point scale, extremely dissatisfied with one point and very satisfied with 5 points. |
Table 1.
Inclusion and Exclusion Criteria
Table 2.
Aronchick Bowel Preparation Scale (ABPS)
Table 3.
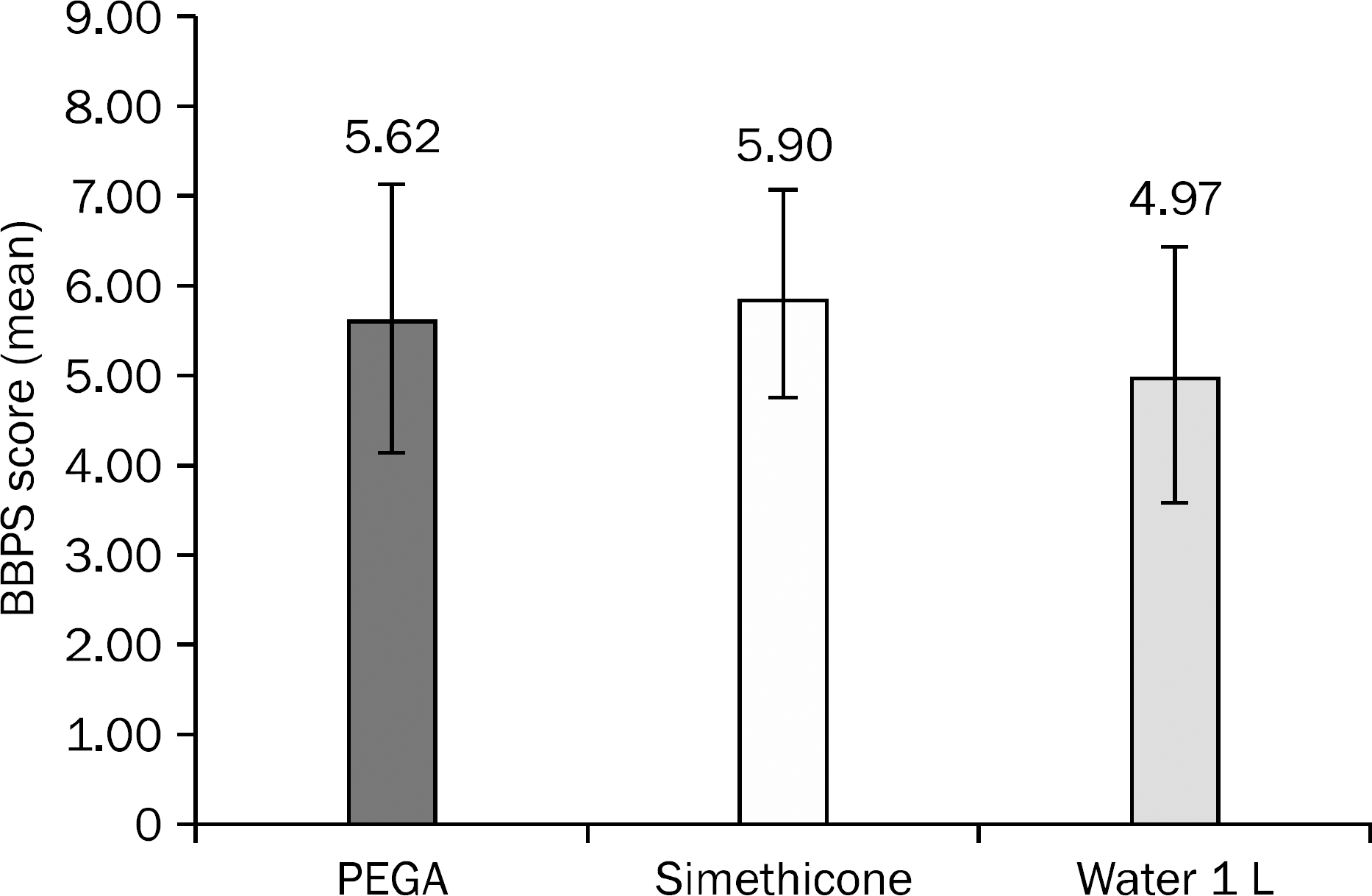
Boston Bowel Preparation Scale (BBPS)
Table 4.
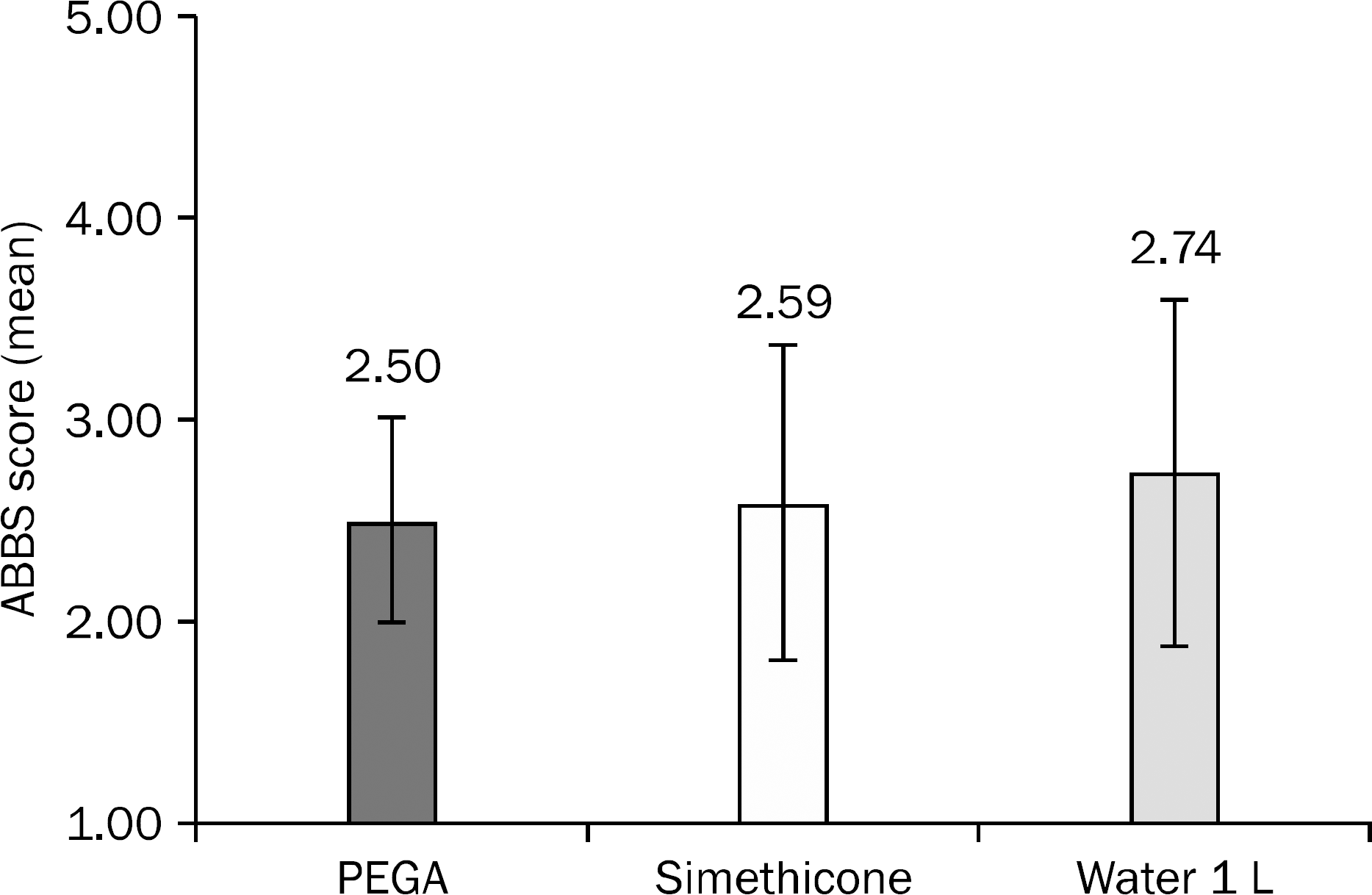
Grading of Intraluminal Air Bubbles
Table 5.
Baseline Characteristics of the Study Groups
Table 6.
Colonoscopic Results and Findings of the Study Groups
Table 7.
Colonoscopic Results, Cleansing Effects and Air Bubbles between the Study Groups
Table 8.
Comparison of Side Effects of the Study Groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download