Abstract
Background/Aims
MicroRNA (miRNA) regulates messenger RNA stability and translation. In cancer biology, miRNA affects the growth and metastasis of cancer cells by controlling epithelial-mesenchymal transition (EMT). MiR-200 family (200a/200b/ 200c/141) and miR-205 are associated with the regulation of EMT. We investigated the prognostic role of EMT-related miRNAs in pancreatic cancer.
Methods
We analyzed miR-200 family and miR-205 expression in tissue samples of 84 patients who underwent radical resection for pancreatic cancer.
Results
Patients were followed from the date of diagnosis until death or censoring. The mean overall survival was 25.0±2.0 months (2–140 months). The R0 resection rate was obtained in 84.5% (n=71) of patients. The relative expressions of miR-200a/200b/200c/141 and miR-205 were 266.9±57.3/18.5±2.2/0.7±0.1/27.2±6.6 folds and 0.1±0.1 compared with human pancreatic ductal epithelial cells, respectively. Overall survival was longer in the low miR-200c expression group than in the high expression group (35 vs. 19 months, p=0.013). Multivariate analysis confirmed that patients with low miR-200c expression survived longer than the high expression group (hazard ratio, 1.771; 95% CI, 1.081–2.900; p=0.023). There was a trend toward longer disease-free survival in low miR-200c group without statistical significance (p=0.061).
References
1. Lai EC. Micro RNAs are complementary to 3' UTR sequence mo-tifs that mediate negative posttranscriptional regulation. Nat Genet. 2002; 30:363–364.

3. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75:843–854.

4. Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001; 107:823–826.
5. Lu M, Zhang Q, Deng M, et al. An analysis of human microRNA and disease associations. PLoS One. 2008; 3:e3420.

6. Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005; 579:5911–5922.

7. Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005; 438:685–689.

8. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005; 435:834–838.

9. Scherr M, Venturini L, Battmer K, et al. Lentivirus-mediated anta-gomir expression for specific inhibition of miRNA function. Nucleic Acids Res. 2007; 35:e149.

10. He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007; 7:819–822.
12. Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006; 6:259–269.

13. Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007; 297:1901–1908.

14. Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008; 25:621–628.

15. Thompson EW, Williams ED. EMT and MET in carcinoma–clinical observations, regulatory pathways and new models. Clin Exp Metastasis. 2008; 25:591–592.

16. Kajiyama H, Shibata K, Terauchi M, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007; 31:277–283.

17. Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006; 12:4147–4153.

18. Hiscox S, Jiang WG, Obermeier K, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006; 118:290–301.
19. Hiscox S, Morgan L, Barrow D, Dutkowskil C, Wakeling A, Nicholson RI. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib ('Iressa', ZD1839). Clin Exp Metastasis. 2004; 21:201–212.

20. Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008; 10:593–601.

21. Abrahamsen HN, Steiniche T, Nexo E, Hamilton-Dutoit SJ, Sorensen BS. Towards quantitative mRNA analysis in paraf-fin-embedded tissues using real-time reverse transcriptasepolymerase chain reaction: a methodological study on lymph nodes from melanoma patients. J Mol Diagn. 2003; 5:34–41.
22. Godfrey TE, Kim SH, Chavira M, et al. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5' nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000; 2:84–91.

23. Doleshal M, Magotra AA, Choudhury B, Cannon BD, Labourier E, Szafranska AE. Evaluation and validation of total RNA extraction methods for microRNA expression analyses in formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2008; 10:203–211.

24. Zhang X, Chen J, Radcliffe T, Lebrun DP, Tron VA, Feilotter H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J Mol Diagn. 2008; 10:513–519.

25. Yu J, Ohuchida K, Mizumoto K, et al. MicroRNA, hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010; 9:169.

26. Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007; 67:8699–8707.

27. Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008; 14:2690–2695.

28. Helleman J, Jansen MP, Burger C, van der Burg ME, Berns EM. Integrated genomics of chemotherapy resistant ovarian cancer: a role for extracellular matrix, TGFbeta and regulating microRNAs. Int J Biochem Cell Biol. 2010; 42:25–30.

29. Eitan R, Kushnir M, Lithwick-Yanai G, et al. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009; 114:253–259.

30. Leskelä S, Leandro-García LJ, Mendiola M, et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer 2010;18: Oncol. 2009; 114:253–259.
Fig. 1.
Overall survival (p=0.013) (A) and disease-free survival (p=0.061) (B) according to the level of microRNA (miRNA)-200c.
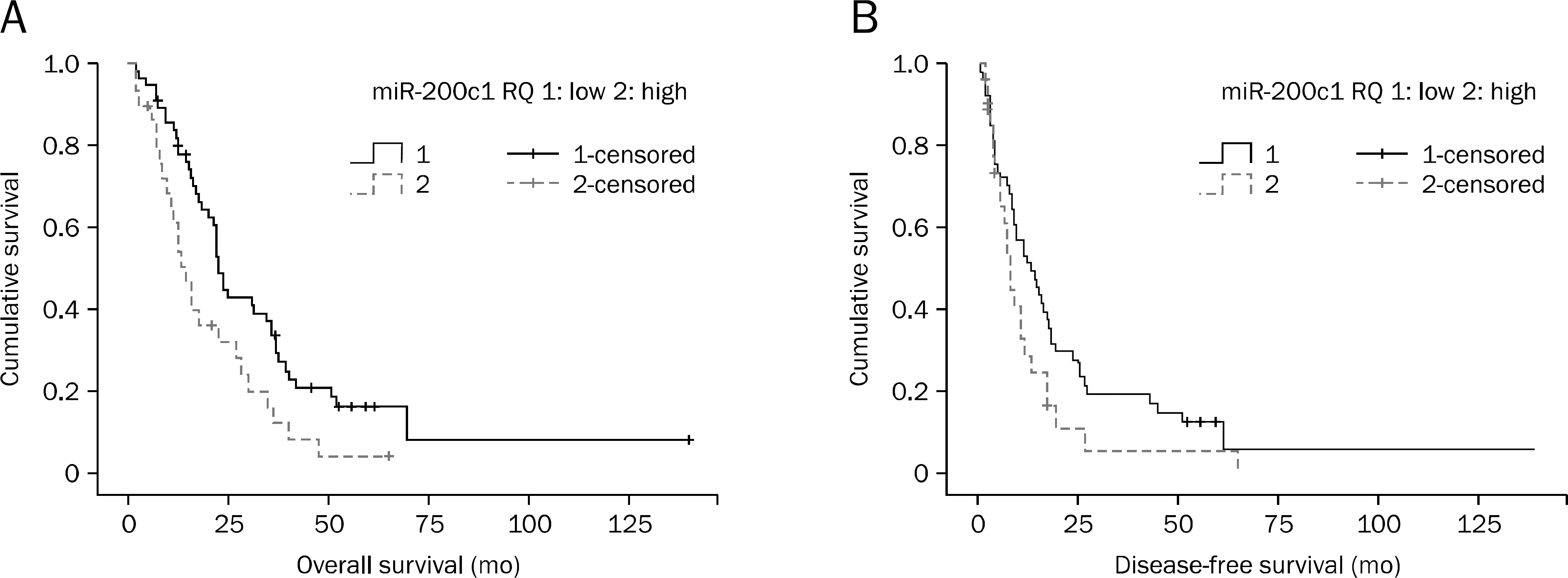
Table 1.
The MicroRNA (miRNA) Primer Sequences for Quantitative Real-time PCR Analysis
Table 2.
Baseline Characteristics of 84 Resected Pancreatic Cancer Patients
Table 3.
MicroRNA (miRNA) Expression Levels in Pancreatic Cancer Tissue Comparing with Immortalized Pancreatic Duct Epithelial Cells
| miRNA | HPDE cells | Mean±SEM |
|---|---|---|
| miRNA-200a | 1 | 266.9±57.3 |
| miRNA-200b | 1 | 18.5±2.2 |
| miRNA-200c | 1 | 0.7±0.1 |
| miRNA-141 | 1 | 27.2±6.6 |
| miRNA-205 | 1 | 0.1±0.1 |
Table 4.
Prognostic Factors of Pancreatic Cancer




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download