Abstract
Background/Aims
Primary non-ampullary duodenal adenocarcinomas (PNADAs) comprise <0.3% of gastrointestinal malignancies. The rarity of PNADA and poorly defined natural history often leads to a delayed correct diagnosis. This study was conducted to evaluate the clinical characteristics of PNADA and to identify its prognostic factors.
Methods
Data were collected by retrospectively reviewing the medical records of patients with PNADA managed at Gyeongsang National University Hospital from January 2000 to December 2014. Demographic, clinical, endoscopic, and pathological variables were investigated, and factors related to survival were analyzed.
Results
Twenty-seven patients with PNADA were identified, and their median age was 64.9±13.6 years with 16 (59.3%) being male. The majority of patients (25/27, 92.6%) were initially diagnosed during upper endoscopy with biopsies. The tumor was located on the 1st or 2nd portion of duodenum in 92.6% (25/27) of patients. At the time of diagnosis, 85.2% (23/27) had advanced diseases (stage III or IV); 48.2% (13/27) had distant metastasis. Median survival time was 12 months (1–93 months). One and 3-year survival rates were 48.1% and 33.3%, respectively. On multivariable analysis, total bilirubin ≥2 mg/dL (OR, 85.28; 95% CI, 3.77–1,938.79; p=0.005) and distant metastasis (OR, 26.74; 95% CI, 3.13–2,328.14; p=0.003) at the time of diagnosis were independent poor prognostic factors.
References
1. Mayer R. Gastrointestinal tract cancer. Longo D, Fauci A, Kasper-Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison's principles of internal medicine: self-assessment and board review. 18th ed.New York: McGraw-Hill;2012.
2. Chung WC, Paik CN, Jung SH, et al. Prognostic factors associated with survival in patients with primary duodenal adenocarcinoma. Korean J Intern Med. 2011; 26:34–40.

3. Barnes G Jr, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994; 1:73–78.

4. Lee CC, Ng WK, Lin KW, Lai TW, Li SM. Adenocarcinoma of the duodenum. Hong Kong Med J. 2008; 14:67–69.
5. Adedeji OA, Trescoli-Serrano C, Garcia-Zarco M. Primary duodenal carcinoma. Postgrad Med J. 1995; 71:354–358.

6. Ahn HS, Jang JY, Lee SE, Yang SH, Lee KU, Kim SW. Surgical treatment and outcomes of primary duodenal adenocarcinoma. J Korean Surg Soc. 2007; 72:38–45.
7. Santoro E, Sacchi M, Scutari F, Carboni F, Graziano F. Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology. 1997; 44:1157–1163.
8. Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997; 46:507–513.

9. Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003; 37:381–386.

10. Takahashi T, Ando T, Kabeshima Y, et al. Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol. 2009; 44:1377–1383.

11. Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008; 40:806–810.

12. Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009; 69:66–73.

13. Endo M, Abiko Y, Oana S, et al. Usefulness of endoscopic treatment for duodenal adenoma. Dig Endosc. 2010; 22:360–365.

14. Kakushima N, Kanemoto H, Sasaki K, et al. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015; 21:5560–5567.

15. Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013; 45:133–135.

16. Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013; 45:136–137.

17. Kakushima N, Ono H, Takao T, Kanemoto H, Sasaki K. Method and timing of resection of superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2014; 26(Suppl 2):35–40.

18. Lee HG, You DD, Paik KY, Heo JS, Choi SH, Choi DW. Prognostic factors for primary duodenal adenocarcinoma. World J Surg. 2008; 32:2246–2252.

19. Hung FC, Kuo CM, Chuah SK, et al. Clinical analysis of primary duodenal adenocarcinoma: an 11-year experience. J Gastroenterol Hepatol. 2007; 22:724–728.

20. Fagniez P, Rotman N. Malignant tumors of the duodenum. Holzheimer R, Mannick J, editors. Surgical treatment: evidencebased and problem-oriented. Munich: Zuckschwerdt;2001.
21. Fernandez-Cruz L. Periampullary carcinoma. Holzheimer R, Mannick J, editors. Surgical treatment: evidencebased and prob-lem-oriented. Munich: Zuckschwerdt;2001.
22. Siewert J, Sendler A. Preoperative staging for gastric cancer. Holzheimer R, Mannick J, editors. Surgical treatment: evidencebased and problem-oriented. Munich: Zuckschwerdt;2001.
23. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. Volume 649. New York: Springer;2010.
24. Kokosis G, Ceppa EP, Tyler DS, Pappas TN, Perez A. Laparoscopic duodenectomy for benign nonampullary duodenal neoplasms. Surg Laparosc Endosc Percutan Tech. 2015; 25:158–162.

25. Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000; 135:1070–1074.
26. Seo JY, Hong SJ, Han JP, et al. Usefulness and safety of endoscopic treatment for nonampullary duodenal adenoma and adenocarcinoma. J Gastroenterol Hepatol. 2014; 29:1692–1698.

27. Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy. 2013; 45:138–141.

28. Cho KH, Jang JY, Kim JY, et al. A case of primary duodenal adenocarcinoma treated by endoscopic mucosal resection. Korean J Gastrointest Endosc. 2010; 40:186–189.
Fig. 1.
Overall survival curve in patients with primary non-ampullary duodenal adenocarcinoma. Overall one-year and 3-year survival rates were 48.1% and 33.3%, respectively in 27 patients with primary non-ampullary duodenal adenocacinoma.
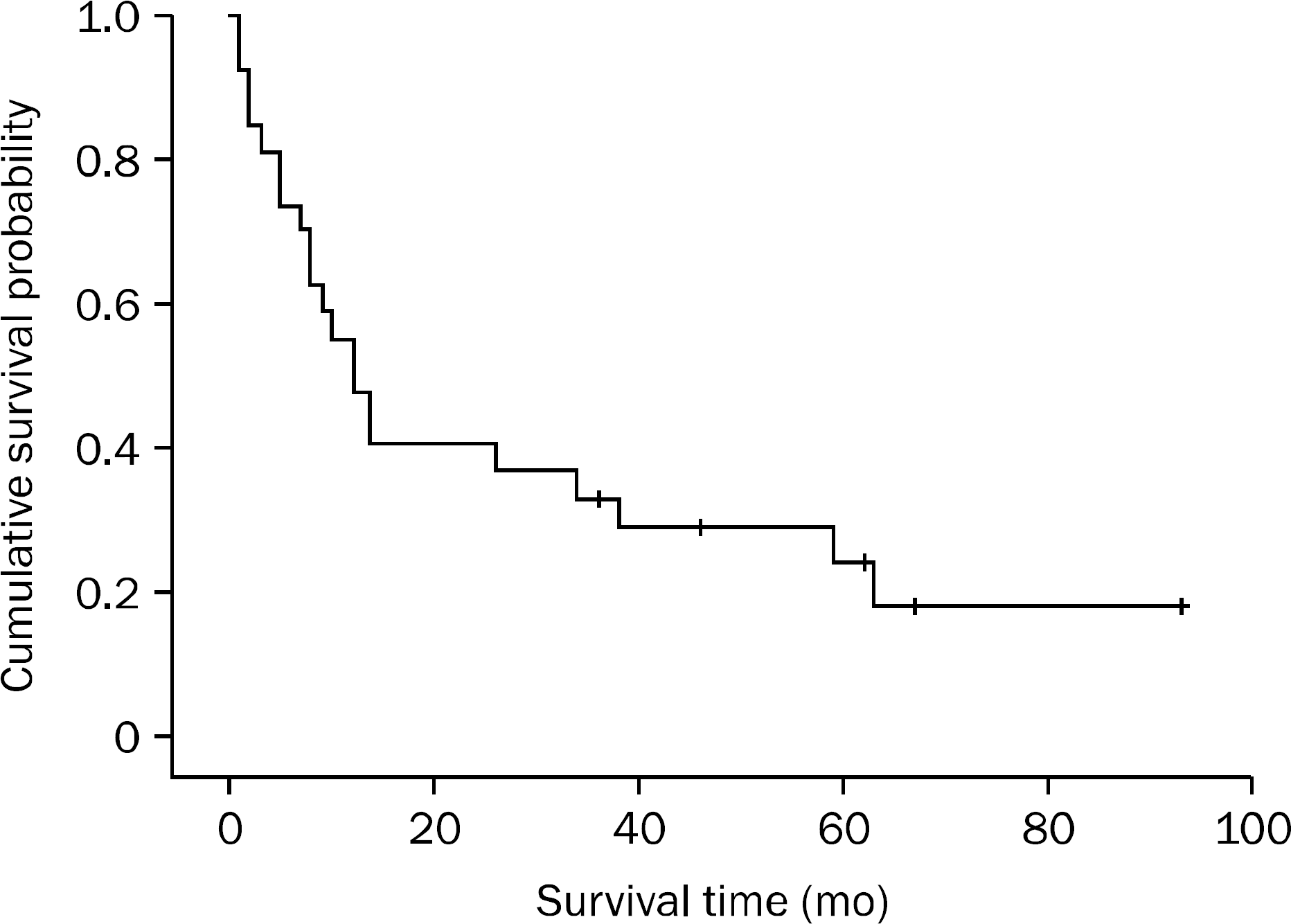
Fig. 2.
Cumulative survival curves in the patients with primary non-ampullary duodenal adenocarcinoma according to total bilirubin and M stage.(A) Survival rate is higher in patients with total bilirubin <2 mg/dL than those with ≥2 mg/dL (p=0.003). (B) Survival rate is higher in M1 than M0 stage (p=0.000).
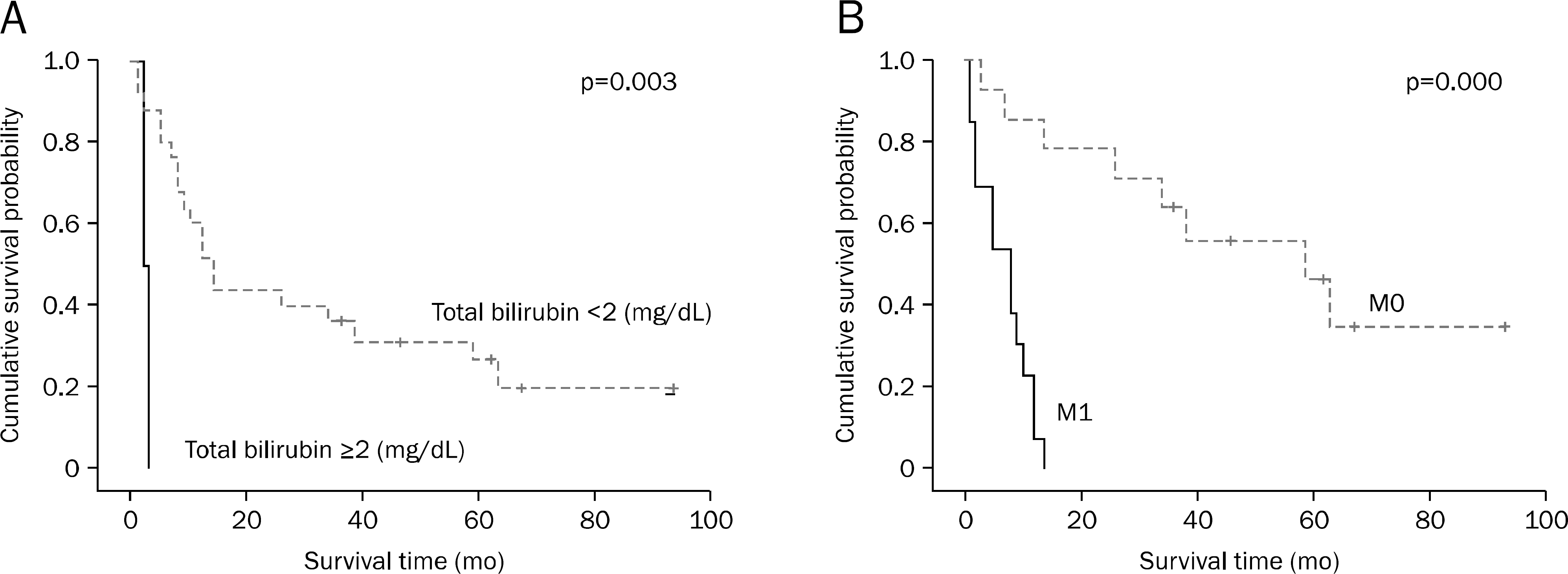
Table 1.
Baseline Characteristics of Patients with Primary non-ampullary Duodenal Adenocarcinoma (n=27)
Table 2.
Summary of 27 Patients with Primary Nonampullary Duodenal Adenocarcinoma
Table 3.
Clinical Outcome of Patients with Nonampullary Primary Duodenal Adenocarcinoma according to Clinical Variables
Table 4.
Prognostic Factors for Survival in Patients with Primary Nonampullary Duodenal Adenocarcinoma on Multivariate Analyses




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download