Abstract
Vasculopathy is rarely reported in neurofibromatosis type 1, but when it occurs it primarily involves the aorta and its main branches. Among vasculopathies, aneurysmal dilatation is the most common form. Although several case reports concerning aneurysms or pseudoaneurysms of visceral arteries in neurofibromatosis type 1 patients have been reported, there are no reports describing gastroduodenal artery aneurysms associated with neurofibromatosis type 1. We experienced a case of life-threatening duodenal ulcer bleeding from a ruptured gastroduodenal artery aneurysm associated with neurofibromatosis type 1. We treated our patient by transarterial embolization after initial endoscopic hemostasis. To our knowledge, this is the first reported case of its type. High levels of suspicion and prompt diagnosis are required to select appropriate treatment options for patients with neurofibromatosis type 1 experiencing upper gastrointestinal bleeding. Embolization of the involved arteries should be considered an essential treatment over endoscopic hemostasis alone to achieve complete hemostasis and to prevent rebleeding.
References
3. Hamilton SJ, Friedman JM. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin Genet. 2000; 58:341–344.

4. Li F, Munchhof AM, White HA, et al. Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet. 2006; 15:1921–1930.

5. Norton KK, Xu J, Gutmann DH. Expression of the neurofibromatosis I gene product, neurofibromin, in blood vessel endothelial cells and smooth muscle. Neurobiol Dis. 1995; 2:13–21.

6. Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol. 2002; 17:562–570. discussion 571–572, 646–651.
7. Dominguez J, Sancho C, Escalante E, Morera JR, Moya JA, Bernat R. Percutaneous treatment of a ruptured intercostal aneurysm presenting as massive hemothorax in a patient with type I neurofibromatosis. J Thorac Cardiovasc Surg. 2002; 124:1230–1232.

9. Brasfield RD, Das Gupta TK. Von Recklinghausen's disease: a clinicopathological study. Ann Surg. 1972; 175:86–104.
10. Kipfer B, Lardinois D, Triller J, Carrel T. Embolization of a ruptured intercostal artery aneurysm in type I neurofibromatosis. Eur J Cardiothorac Surg. 2001; 19:721–723.

11. Mendonça CT, Weingartner J, de Carvalho CA, Costa DS. Endovascular treatment of contained rupture of a superior mesenteric artery aneurysm resulting from neurofibromatosis type I. J Vasc Surg. 2010; 51:461–464.

12. Tsutsumi M, Kazekawa K, Tanaka A, et al. Rapid expansion of benign scalp neurofibroma caused by massive intratumoral hemorrhage: case report. Neurol Med Chir (Tokyo). 2002; 42:338–340.

13. Zhang CW, Yang ZG, Xie XD, Wang CH, You C, Li W. Transcatheter embolization of a ruptured internal pudendal artery pseudoaneurysm in a patient with neurofibromatosis type 1. J Korean Med Sci. 2010; 25:638–640.

Fig. 2.
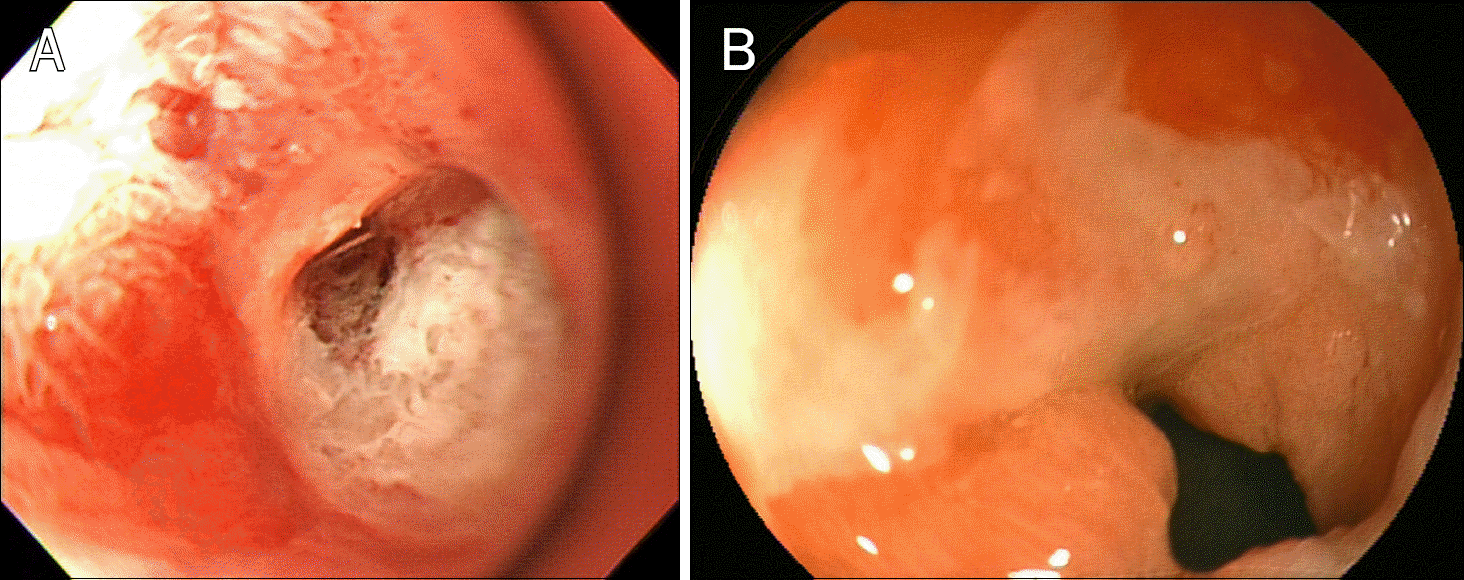
Endoscopy upon admission reveals a small ulcer (3×7 mm) in the duodenal bulb with visible vessel.
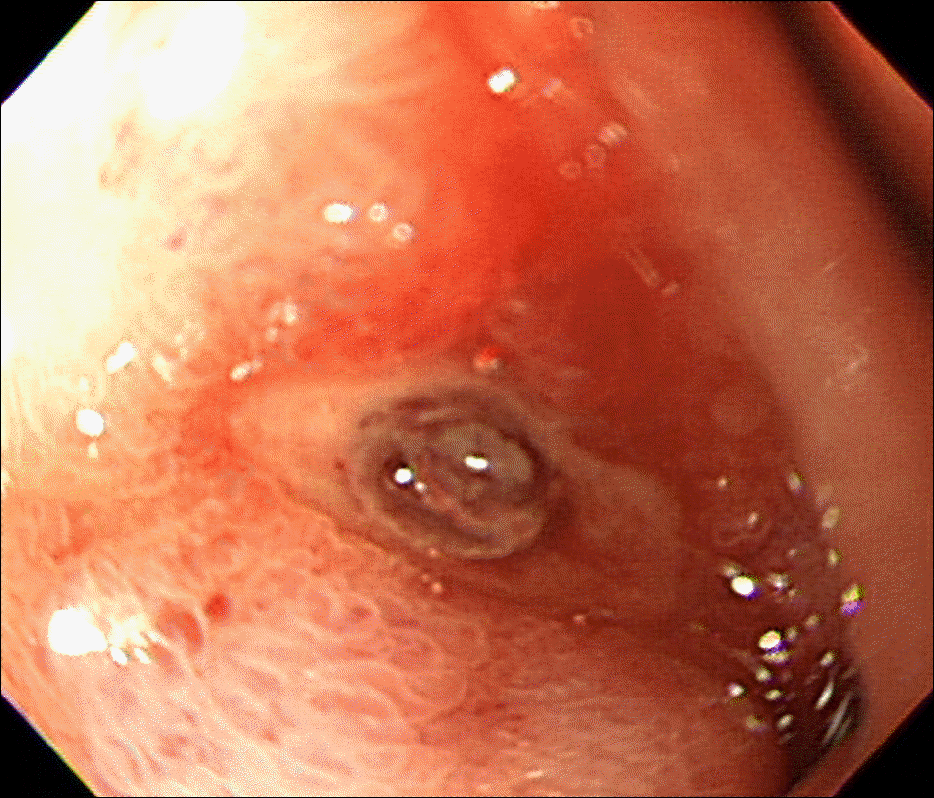
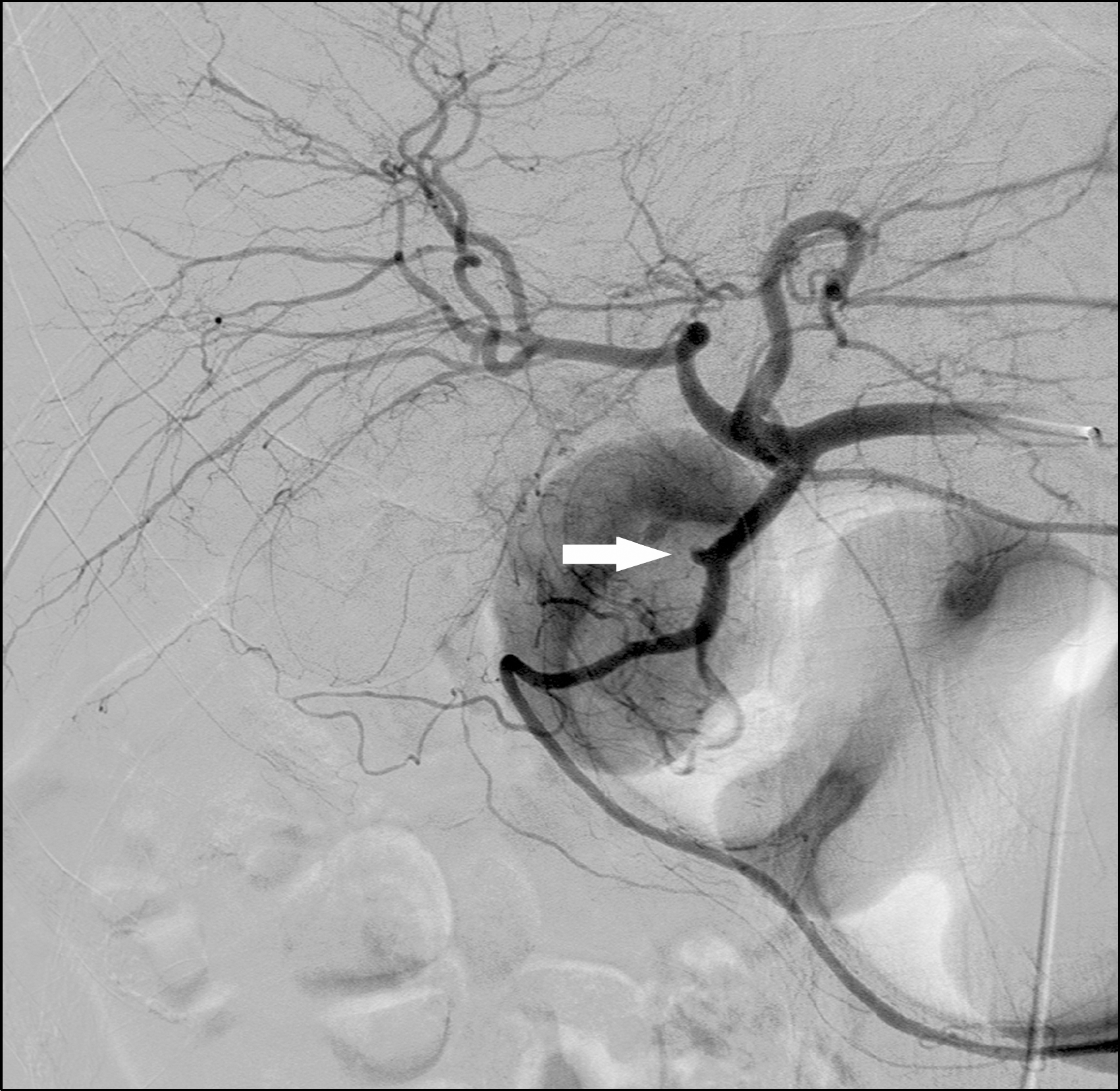
Fig. 3.
Selective angiography of the common hepatic artery shows an aneurysm (arrow) in the mid-portion of the gastroduodenal artery and contrast extravasation into the duodenal lumen before embolization.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


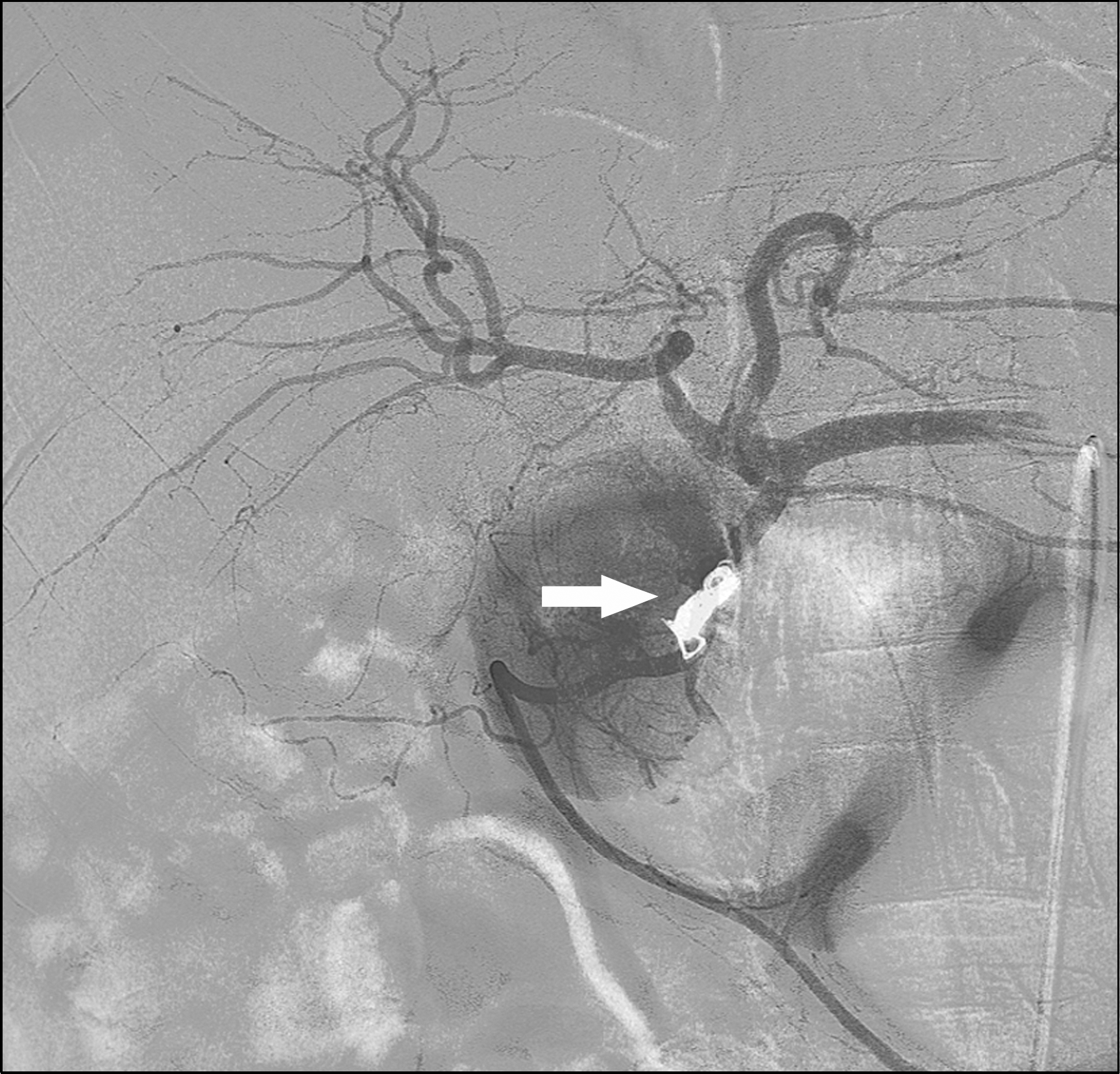
 XML Download
XML Download