Abstract
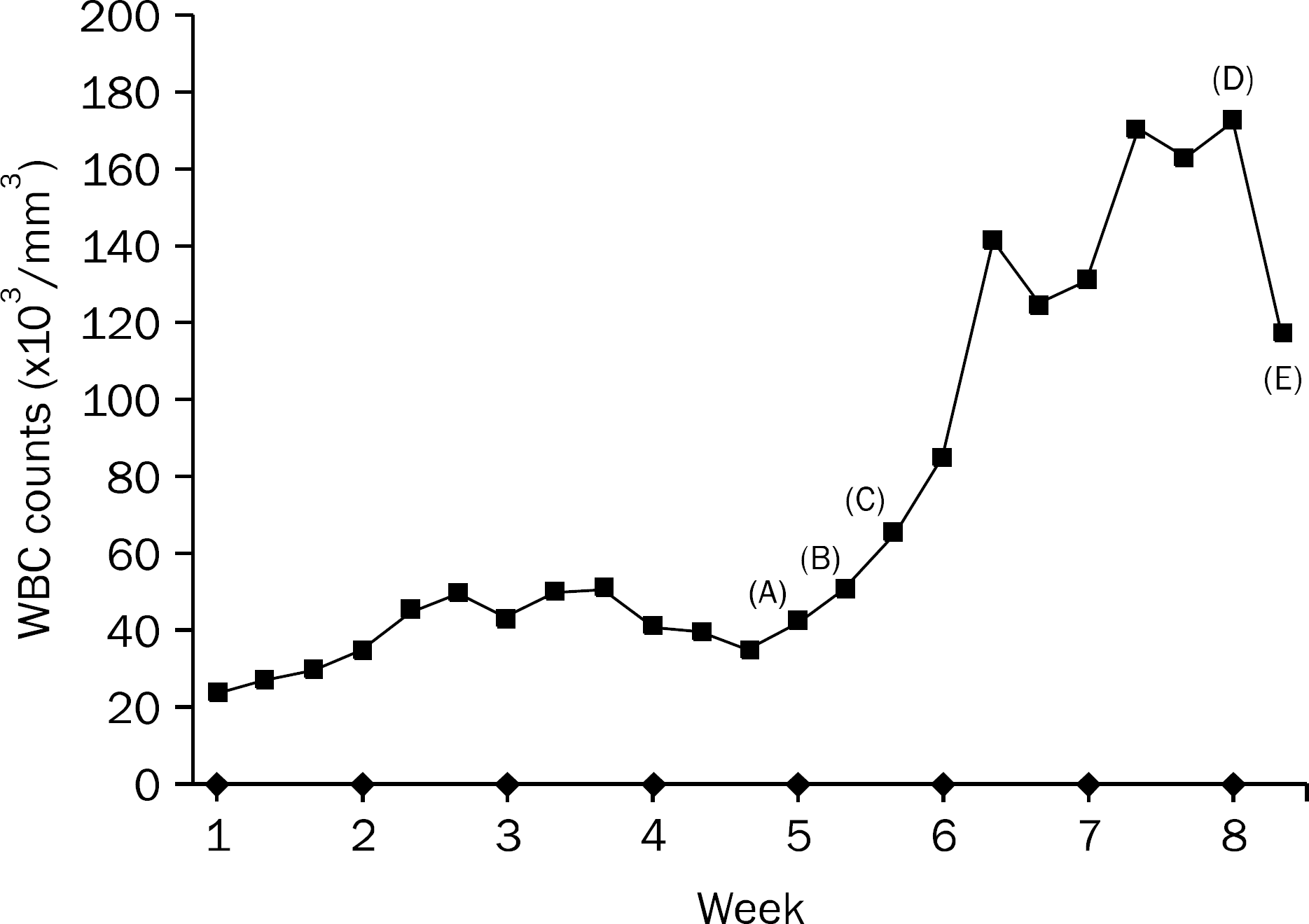
Leukemoid reaction is defined as leukocytosis exceeding 50,000 cells/mm3. When it occurs in a patient with a malignancy, secondary causes such as infections, drugs, hematologic diseases and hemorrhage need to be ruled out. After excluding such causes, paraneoplastic leukemoid reaction can be considered as a diagnosis of exclusion. Paraneoplastic leukemoid reactions have been described in association with lung, gastrointestinal, genitourinary and head and neck cancers. However, pancreatic cancer with leukemoid reaction has been rarely reported. We diagnosed a case of a 55-year-old Korean woman with extreme leukocytosis associated with advanced pancreatic cancer.
References
1. Cancer Statistics Korea 2012–2013. [Internet]. Goyang: National Cancer Information Center;2014 Dec 24. [cited 2015 Feb 1]. Available from:. http://www.cancer.go.kr.
2. Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010; 85:838–854.

3. Sakka V, Tsiodras S, Giamarellos-Bourboulis EJ, Giamarellou H. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur J Intern Med. 2006; 17:394–398.

4. Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009; 115:3919–3923.
5. Godquin B. Cancer of the pancreas and paraneoplastic leukemoid syndrome. Chirurgie. 1973; 98:785–787.
6. Akoun G, Duhamel G, Duvaldestin P, Koubi G, Brocard H. Myeloid leukemoid reaction in cancer of the pancreas: paraneoplastic syndrome or myeloid leukosis? Sem Hop. 1972; 48:861–863.
7. Akoun G, Duhamel G, Duvaldestin P, Koubi G, Brocard H. Paraneoplastic leukemoid reaction of a myeloid type or myeloid leukemia in cancer of the pancreas? Presse Med. 1971; 79:2327.
8. Qureshi KM, Raman AK, Tan D, Fakih MG. Leukemoid reaction in pancreatic cancer: a case report and review of the literature. JOP. 2006; 7:631–634.
9. Ashdhir P, Jain P, Pokharna R, Nepalia S, Sharma SS. Pancreatic cancer manifesting as liver metastases and eosinophillic leukemoid reaction: a case report and review of literature. Am J Gastroenterol. 2008; 103:1052–1054.

10. Mintzer DM, Billet SN, Chmielewski L. Drug-induced hematologic syndromes. Adv Hematol. 2009; 2009:495863.

11. Wetzler M, Estrov Z, Talpaz M, Markowitz A, Gutterman JU, Kurzrock R. Granulocyte-macrophage colony-stimulating factor as a cause of paraneoplastic leukaemoid reaction in advanced transitional cell carcinoma. J Intern Med. 1993; 234:417–420.

12. Sato K, Terada K, Sugiyama T, et al. Granulocyte colony-stimulating factor produced by bladder carcinoma of a patient with leukemoid reaction did not affect proliferation of the tumor cells. J Urol. 1994; 151:1687–1690.

13. Nakada T, Sato H, Inoue F, Mizorogi F, Nagayama K, Tanaka T. The production of colony-stimulating factors by thyroid carcinoma is associated with marked neutrophilia and eosinophilia. Intern Med. 1996; 35:815–820.

14. Chen YM, Whang-Peng J, Liu JM, et al. Leukemoid reaction resulting from multiple cytokine production in metastatic mucoepidermoid carcinoma with central necrosis. Jpn J Clin Oncol. 1995; 25:168–172.
15. Haque A, Aan N. Leukemoid reaction: unusual causes. Int J Pathology. 2010; 8:39–40.
Fig. 1.
A 5.5 cm-sized lobulating contour mass with inner cystic area in pancreatic tail with renal and splenic involvement was noted in pancreas dynamic MRI (A) and in whole body PET-CT (maximum standardized uptake value=6.0) (B).
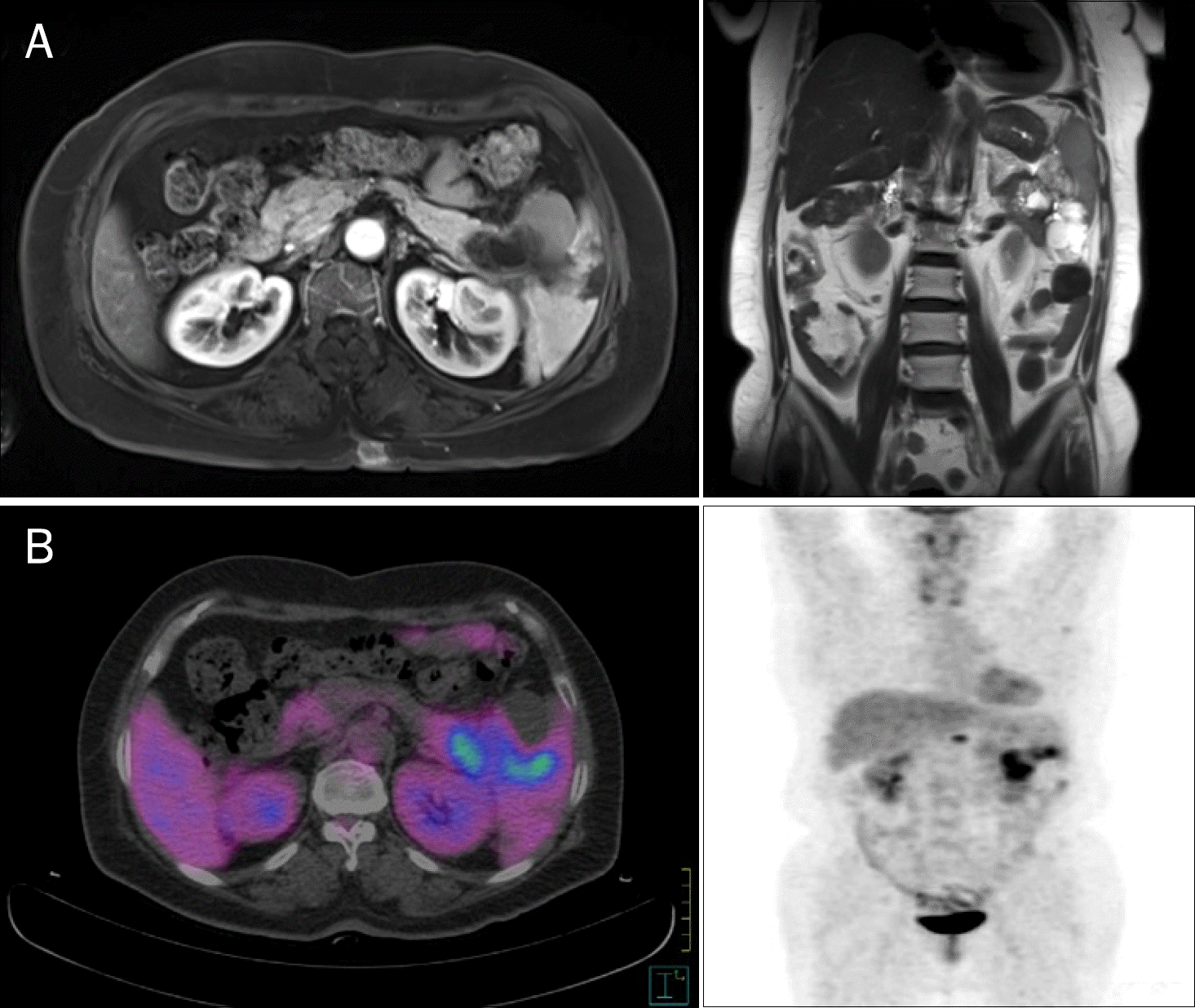
Fig. 2.
Product of distal pancreatectomy, splenectomy, and left nephrectomy. A large irregular solid mass (6.0×4.2×4.0 cm) was found at distal pancreas.
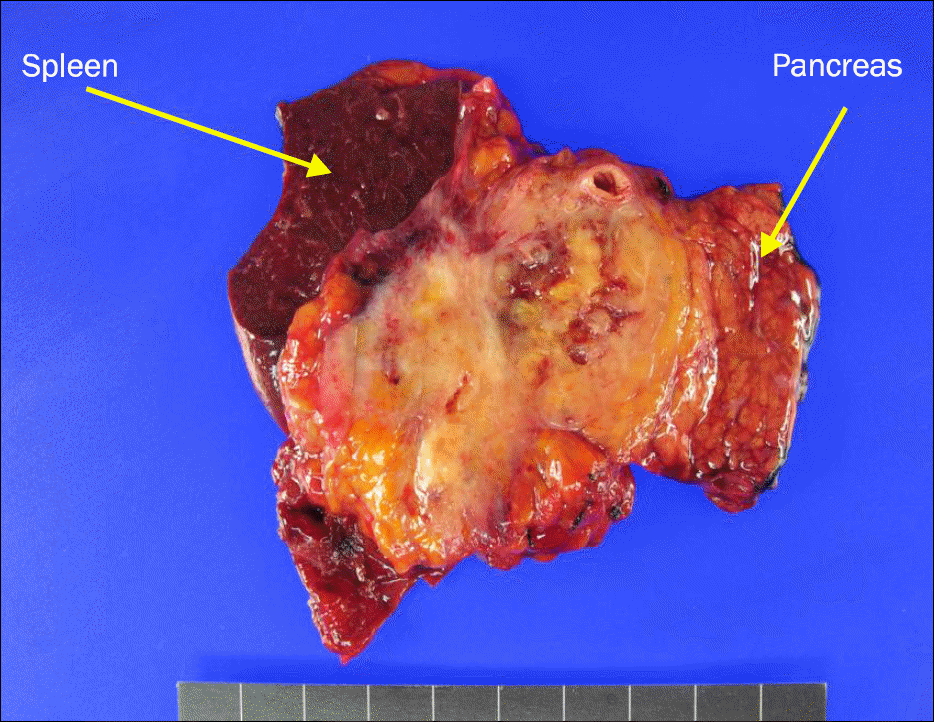
Fig. 3.
On histologic examination, poorly differentiated adenocarcinoma (A) with signet ring cell feature (H&E, ×100; inset: ×400) and mucinous cystic component (B) was noticed (H&E, ×100; inset: ×400).
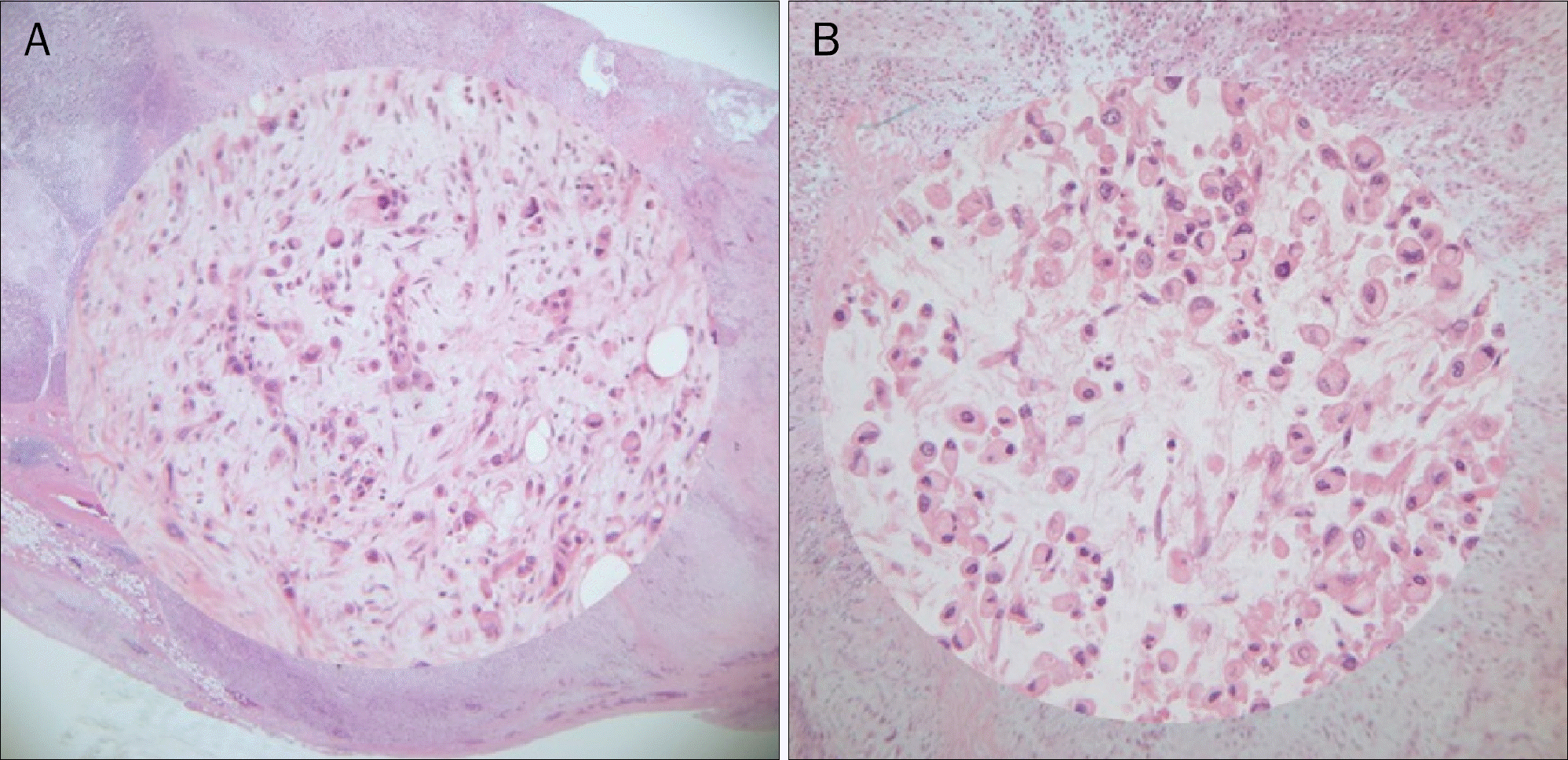
Fig. 4.
Pancreas CT shows multiple hyperechoic lesions with hypoechoic rim (arrows) in both lobes of the liver suggesting multiple metastases to the liver.
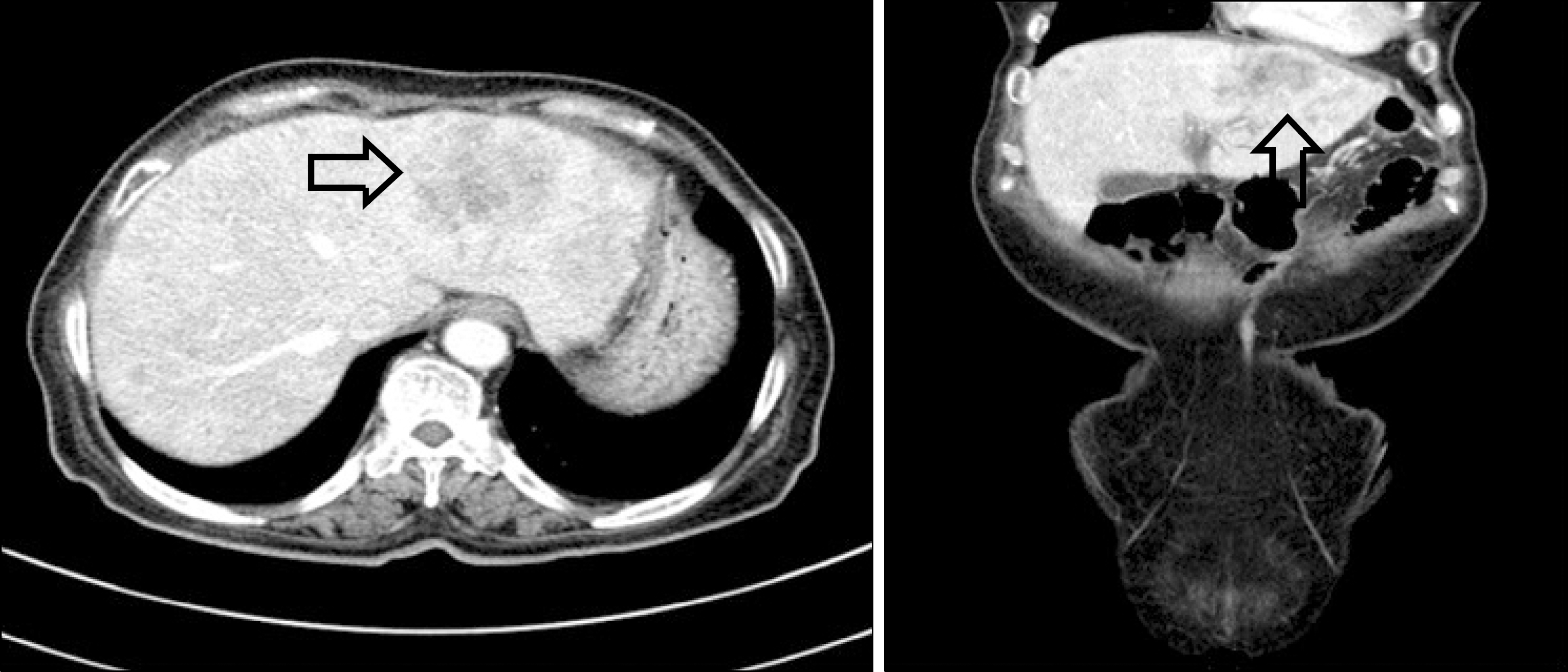
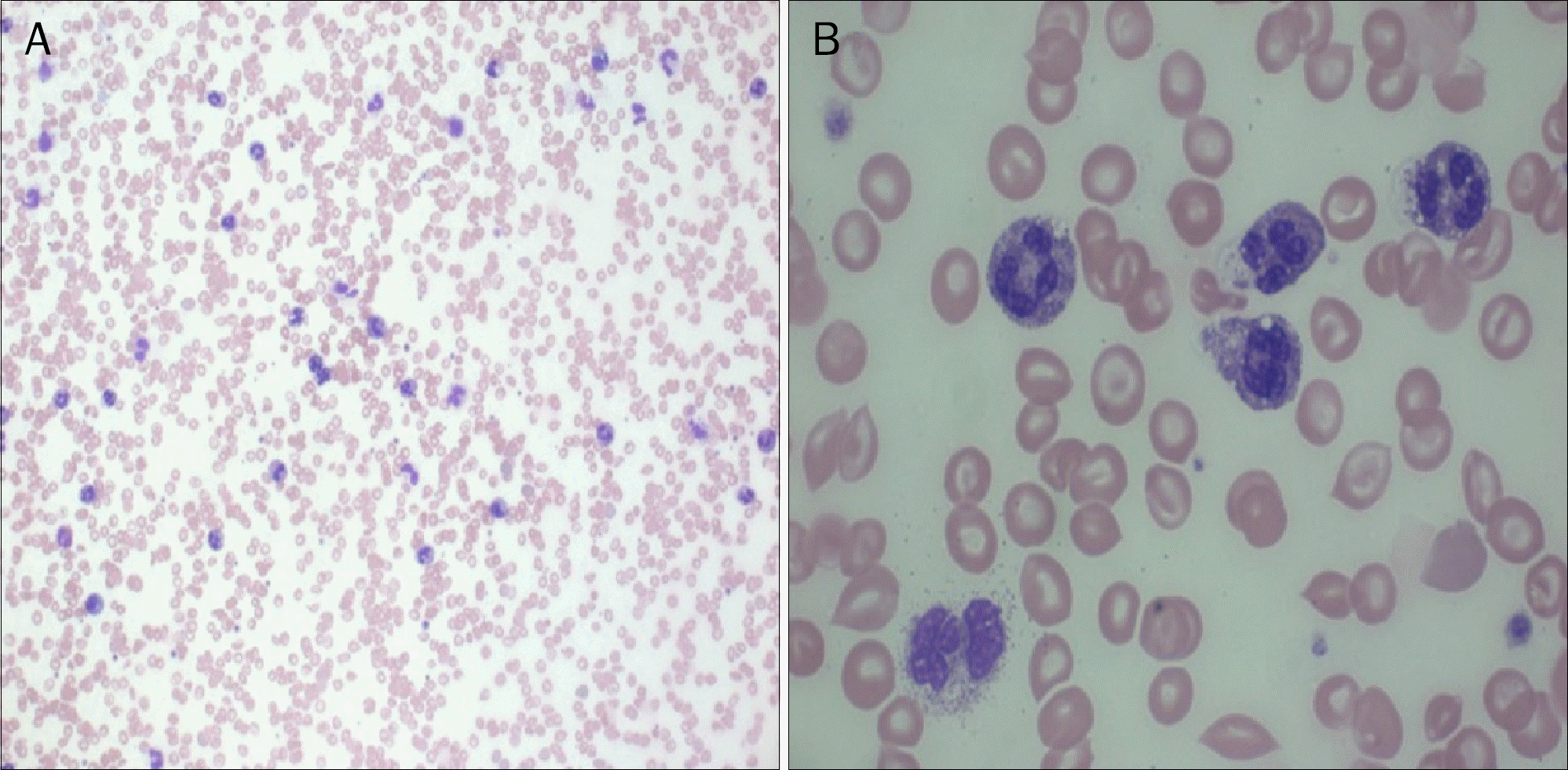
Fig. 5.
Peripheral blood smear reveals marked granulocytosis without bands, myelocytes or metamyelocytes (H&E; A, ×200; B, ×1,000).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download