Abstract
Background/Aims
The purpose of this study was to investigate the efficacy and safety of irinotecan based FOLFIRI chemotherapy as a second-line treatment after failure of FOLFOX-4 chemotherapy in patients with advanced gastric cancer.
Methods
Fifty-two patients who were pathologically diagnosed with unresectable gastric cancer and received FOLFIRI chemotherapy after failure of FOLFOX-4 chemotherapy between September 2005 and February 2012 were enrolled in this study. Data were collected by retrospectively reviewing the medical records. The response to chemotherapy was assessed every 3 cycles by World Health Organization criteria and long term survival was analyzed. The toxicities were evaluated for every course of chemotherapy according to National Cancer Institution (NCI) toxicity criteria version 3.0.
Results
Median age of the patients was 57 years. Median overall survival (OS) and time to progression (TTP) were 7.8 and 5 months, respectively. The number of patients showing complete remission, partial remission, stable disease, and progressive disease were 0 (0.0%), 9 (17.3%), 30 (57.7%), and 13 (25.0%), respectively. The overall response rate was 17.3%. During a total of 345 cycles, anemia worse than NCI toxicity grade 3 occurred in 2.9%, leukopenia in 20.3%, neutropenia in 12.2%, and thrombocytopenia in 1.5%. Patients with less organ involvement by metastasis, less than 34 U/mL of CA 19–9 and good responsiveness to third cycle of second line chemotherapy were associated with longer OS and TTP.
Conclusions
FOLFIRI chemotherapy has a modest efficacy with acceptable toxicities in patients with advanced gastric cancer as a second-line treatment. Further well-controlled studies are needed to elucidate the efficacy of FOLFIRI chemotherapy as second-line treatment in patients with advanced stomach cancer.
Go to : 
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

2. Janunger KG, Hafström L, Nygren P, Glimelius B. SBU-Group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001; 40:309–326.
3. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41.

4. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

5. Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168.

6. Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591.

7. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and metaanalysis based on aggregate data. J Clin Oncol. 2006; 24:2903–2909.

8. Lee CW, Park MI, Park SJ, et al. FOLFOX-4 combination chemotherapy as a first-line treatment in patients with advanced gastric cancer. Korean J Med. 2012; 82:37–44.

9. Kim JA, Lee J, Han B, et al. Docetaxel/cisplatin followed by FOLFIRI versus the reverse sequence in metastatic gastric cancer. Cancer Chemother Pharmacol. 2011; 68:177–184.

10. Lee KW, Kim JH, Yun T, et al. Phase II study of low-dose paclitaxel and cisplatin as a second-line therapy after 5-fluorouracil/platinum chemotherapy in gastric cancer. J Korean Med Sci. 2007; 22(Suppl):S115–S121.

11. Wilson D, Hiller L, Geh JI. Review of second-line chemotherapy for advanced gastric adenocarcinoma. Clin Oncol (R Coll Radiol). 2005; 17:81–90.

12. Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011; 47:2306–2314.
13. Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pre-treated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012; 30:1513–1518.

14. Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet Oncol. 2009; 10:903–912.

15. Jung JT. Chemotherapy of advanced gastric cancer. Korean J Helicobacter Up Gastrointest Res. 2013; 13:142–146.

16. Lorizzo K, Fazio N, Radice D, et al. Simplified FOLFIRI in pre-treated patients with metastatic gastric cancer. Cancer Chemother Pharmacol. 2009; 64:301–306.

17. Kim ST, Kang WK, Kang JH, et al. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005; 92:1850–1854.

18. Jeon EK, Hong SH, Kim TH, et al. Modified FOLFIRI as second-line chemotherapy after failure of modified FOLFOX-4 in advanced gastric cancer. Cancer Res Treat. 2011; 43:148–153.

19. Seo MD, Lee KW, Lim JH, et al. Irinotecan combined with 5-fluorouracil and leucovorin as second-line chemotherapy for metastatic or relapsed gastric cancer. Jpn J Clin Oncol. 2008; 38:589–595.

20. Park JS, Lim JY, Park SK, et al. Prognostic factors of second and third line chemotherapy using 5-fu with platinum, irinotecan, and taxane for advanced gastric cancer. Cancer Res Treat. 2011; 43:236–243.

21. Seymour MT, Maughan TS, Ledermann JA, et al. FOCUS Trial Investigators; National Cancer Research Institute Colorectal Clinical Studies Group. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007; 370:143–152.

Go to : 
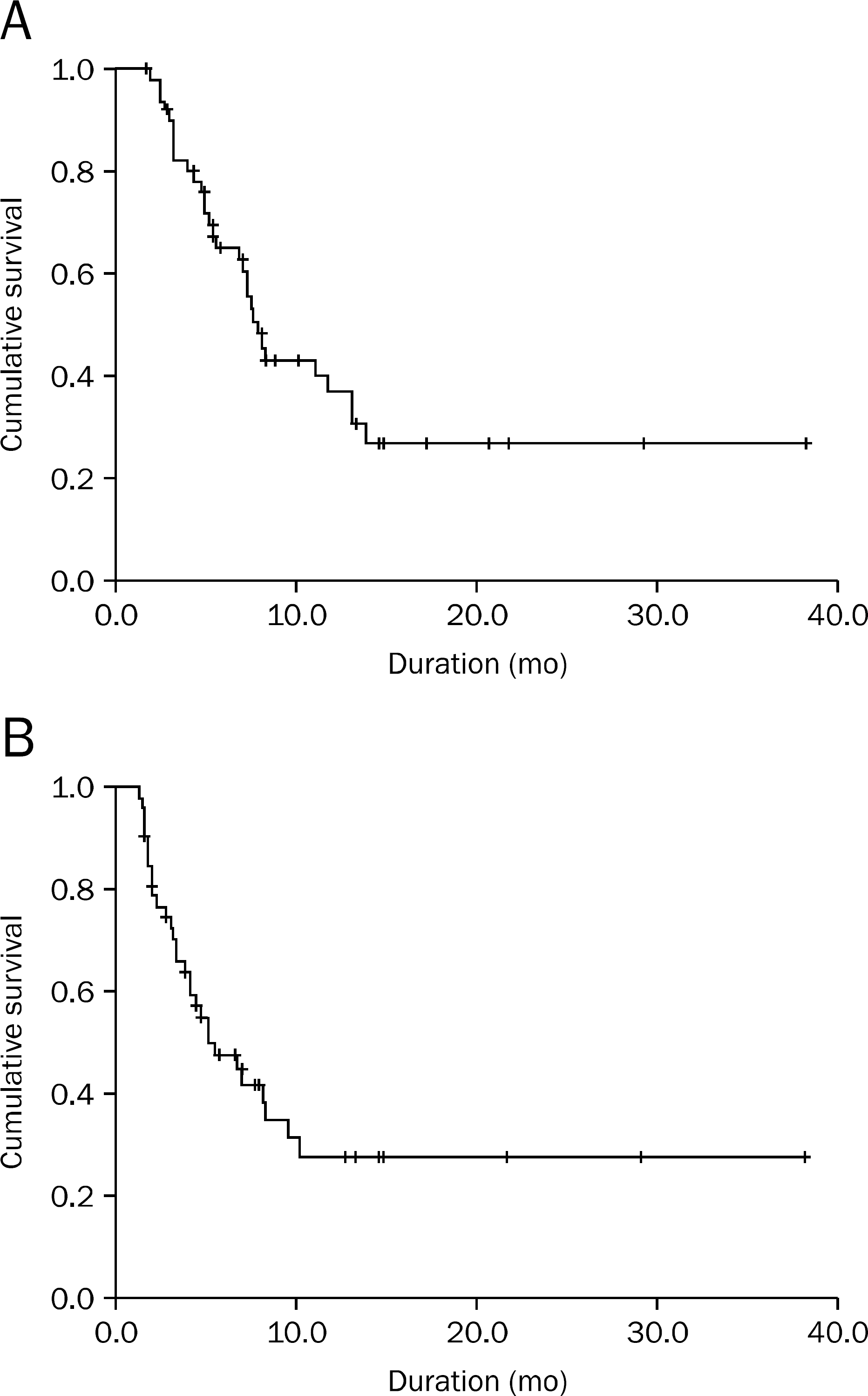 | Fig. 1.Overall survival and progression free survival of patients after FOLFIRI combination therapy. (A) The median overall survival was 7.8 months (95% CI, 6.6–9.0). (B) The median time to progression was 5 months (95% CI, 2.3–7.7). |
Table 1.
Baseline Characteristics
Table 2.
Treatment Response to FORFIRI Chemotherapy
| Response a | Patient |
|---|---|
| Complete remission | 0 (0) |
| Partial remission | 9 (17.3) |
| Stable disease | 30 (57.7) |
| Progressive disease | 13 (25.0) |
| Response rate | 17.3 |
| Disease control rate | 75.0 |
Table 3.
Prognostic Factor according to Univariate Analysis
Table 4.
Prognostic factor according to Cox Multivariate Analysis
Table 5.
Toxicity Profiles of FOLFIRI (per Patient)
Table 6.
Comparison with Other Studies Using Irinotecan-based Chemotherapy in Korea
| Variable | Kim et al.17 | Seo et al.19 | Jeon et al.18 | Our study |
|---|---|---|---|---|
| Irinotecan, LV and 5-FU regimens (mg/m2) | 150, 100, 1,000 a | 180, 200, 400 b+600 a | 150, 200, 400 b+600 a | 160 or 180, 200, 400 b+600 a |
| Patient (n) | 64 | 51 | 32 | 52 |
| Median age (yr) | 55 | 55 | 59 | 57 |
| Response rate (%) | 21 | 18 | 9.4 | 17.3 |
| Median overall survival (mo) | 7.6 | 9.1 | 5.8 | 7.8 |
| Median time to progression (mo) | 2.5 | 3.2 | 2 | 5 |
| Grade 1–2 neutropenia (%) | 11 | 37.5 | 47.0 | |
| Grade 3–4 neutropenia (%) | 11 | 17 | 21.9 | 12.2 |
| Grade 3–4 anemia (%) | 4 | 6.2 | 2.9 | |
| Grade 3–4 thrombocytopenia (%) | 3 | 2 | 3.1 | 1.5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download